Deck 21: Environmental Chemistry Earths Environment,energy,and Sustainability
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/48
Play
Full screen (f)
Deck 21: Environmental Chemistry Earths Environment,energy,and Sustainability
1
Helium and argon are added continuously to the atmosphere.What is the primary source of these gases?
A) the solar wind
B) mining operations
C) burning of fossil fuels
D) radioactive decay
E) volcanic eruptions
A) the solar wind
B) mining operations
C) burning of fossil fuels
D) radioactive decay
E) volcanic eruptions
radioactive decay
2
Which water pollutant can lead to an increased occurrence in skin lesions and skin cancer when ingested at unsafe (toxic)levels?
A) arsenic
B) copper
C) mercury
D) fluoride
E) lead
A) arsenic
B) copper
C) mercury
D) fluoride
E) lead
arsenic
3
Which water pollutant is responsible for algal blooms?
A) arsenic
B) phosphates
C) mercury
D) fluoride
E) trihalomethanes
A) arsenic
B) phosphates
C) mercury
D) fluoride
E) trihalomethanes
phosphates
4
The minimum wavelength of light required to decompose a single ozone molecule is 320.nm.Calculate the minimum energy required to decompose 1.00 mole of ozone.(h = 6.626 10-34 J.s; c = 3.00 108 m/s; 1 nm = 10-9 m; 1mol = 6.022 1023)
A) 6.21 10-19 kJ/mol
B) 120 kJ/mol
C) 0.374 kJ/mol
D) 374 kJ/mol
E) 0.00267 kJ/mol
A) 6.21 10-19 kJ/mol
B) 120 kJ/mol
C) 0.374 kJ/mol
D) 374 kJ/mol
E) 0.00267 kJ/mol

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following is not responsible for the formation and/or release of methane into the atmosphere?
A) aerobic bacteria
B) cows
C) landfills
D) mining
E) termites
A) aerobic bacteria
B) cows
C) landfills
D) mining
E) termites

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is the main form of nitrogen absorbed by plants?
A) NH3
B) NH4+
C) NO3-
D) N2
E) NO2
A) NH3
B) NH4+
C) NO3-
D) N2
E) NO2

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
7
Which gas has the highest concentration in the Earth's atmosphere?
A) N2
B) O2
C) Ar
D) CO2
E) O3
A) N2
B) O2
C) Ar
D) CO2
E) O3

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
8
The conversion (fixation)of N2 to its compounds is accomplished by a number of natural and artificial processes.Which of the following account for around 50% (the highest amount)of worldwide conversion of nitrogen to its compounds?
A) burning fossil fuels
B) lightening
C) biological processes
D) fertilizer production
E) volcanic eruptions
A) burning fossil fuels
B) lightening
C) biological processes
D) fertilizer production
E) volcanic eruptions

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
9
All of the following are used by various cities and municipalities to disinfect water except ___.
A) Cl2
B) NaOCl
C) O3
D) UV-light
E) O2
A) Cl2
B) NaOCl
C) O3
D) UV-light
E) O2

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following water disinfectants persist in water pipes even after the initial disinfection process?
A) Cl2
B) NaOCl
C) O3
D) A and B
E) A,B,and C
A) Cl2
B) NaOCl
C) O3
D) A and B
E) A,B,and C

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
11
The mechanism of the chlorine atom catalyzed decomposition of ozone is as follows.
O3 + Cl -ClO + O2
-ClO + -O -Cl + O2
O3 + UV light -O + O2
Identify any intermediates in the mechanism.
A) O3
B) (-Cl)
C) (-ClO)
D) O2
E) (-O) and (-Cl)
O3 + Cl -ClO + O2
-ClO + -O -Cl + O2
O3 + UV light -O + O2
Identify any intermediates in the mechanism.
A) O3
B) (-Cl)
C) (-ClO)
D) O2
E) (-O) and (-Cl)

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
12
The largest source of electricity production in the United States is from ___.
A) wind
B) hydroelectricity
C) solar
D) geothermal
E) biomass
A) wind
B) hydroelectricity
C) solar
D) geothermal
E) biomass

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
13
What is the correct name for NO3-?
A) nitric oxide
B) nitrite
C) nitrogen trioxide
D) nitrous oxide
E) nitrate
A) nitric oxide
B) nitrite
C) nitrogen trioxide
D) nitrous oxide
E) nitrate

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
14
What compound was used by the ancient Egyptians and is still used today in the removal of visible impurities (cloudiness)in water?
A) sand (SiO2)
B) aluminum(III)hydroxide (Al(OH)3)
C) slaked lime (Ca(OH)2)
D) alum (KAl(SO4)2)
E) chlorine (Cl2)
A) sand (SiO2)
B) aluminum(III)hydroxide (Al(OH)3)
C) slaked lime (Ca(OH)2)
D) alum (KAl(SO4)2)
E) chlorine (Cl2)

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
15
The majority of electricity generated in the United States is produced from ___.
A) wind and solar
B) moving water
C) nuclear fission
D) natural gas
E) coal
A) wind and solar
B) moving water
C) nuclear fission
D) natural gas
E) coal

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
16
Which ion occurs in the highest concentration in seawater?
A) Cl-
B) Na+
C) HCO3-CO32-
D) Mg2+
E) Ca2+
A) Cl-
B) Na+
C) HCO3-CO32-
D) Mg2+
E) Ca2+

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
17
What is the most abundant nitrogen oxide in the atmosphere?
A) NO
B) NO2
C) N2O
D) NO3-
E) NO2-
A) NO
B) NO2
C) N2O
D) NO3-
E) NO2-

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
18
What is the electron geometry around the central oxygen in O3?
A) linear
B) bent
C) trigonal planar
D) tetrahedral
E) trigonal bipyramidal
A) linear
B) bent
C) trigonal planar
D) tetrahedral
E) trigonal bipyramidal

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is considered a renewable source of energy?
A) solar energy
B) hydroelectric power
C) nuclear fission
D) wind power
E) geothermal energy
A) solar energy
B) hydroelectric power
C) nuclear fission
D) wind power
E) geothermal energy

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
20
Desalination of seawater by reverse osmosis requires application of a pressure in excess of the solutions osmotic pressure.What is the osmotic pressure of a 5.0 g NaCl/L solution at 25 C? (assume the van't Hoff factor i = 2; R = 0.0826 L.atm/mol.K)
A) 4.2 atm
B) 2.1 atm
C) 0.35 atm
D) 250 atm
E) 21 atm
A) 4.2 atm
B) 2.1 atm
C) 0.35 atm
D) 250 atm
E) 21 atm

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
21
Many homes are heated using natural gas.The combustion of natural gas converts
A) chemical potential energy to thermal energy.
B) thermal energy to mechanical energy.
C) mechanical energy to chemical potential.
D) electrostatic energy to mechanical energy.
E) gravitational energy to acoustic energy.
A) chemical potential energy to thermal energy.
B) thermal energy to mechanical energy.
C) mechanical energy to chemical potential.
D) electrostatic energy to mechanical energy.
E) gravitational energy to acoustic energy.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
22
What mass of sulfur dioxide gas would be produced from 6.00 kg of coal which contains 1.15 % sulfur by mass?
A) 276 g
B) 12.0 g
C) 34.5 g
D) 138 g
E) 103 g
A) 276 g
B) 12.0 g
C) 34.5 g
D) 138 g
E) 103 g

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
23
Most hydrogen gas is produced industrially by
A) electrolysis.
B) solar energy.
C) steam reforming.
D) bacteria.
E) fuel cells.
A) electrolysis.
B) solar energy.
C) steam reforming.
D) bacteria.
E) fuel cells.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
24
What process is used to covert coal into coke?
A) steam reforming
B) liquefaction
C) fermentation
D) heating in the absence of air
E) heating in air
A) steam reforming
B) liquefaction
C) fermentation
D) heating in the absence of air
E) heating in air

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
25
What form of radiation is trapped by greenhouse gases?
A) microwaves
B) UV radiation
C) visible radiation
D) infrared radiation
E) radio waves
A) microwaves
B) UV radiation
C) visible radiation
D) infrared radiation
E) radio waves

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following cannot be used in the production of bioethanol?
A) cellulose from grasses
B) sugar beets
C) sugar cane
D) corn
E) natural gas
A) cellulose from grasses
B) sugar beets
C) sugar cane
D) corn
E) natural gas

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following is a component of petroleum?
A) alkynes
B) alkenes
C) alkanes
D) A and B
E) A,B,and C
A) alkynes
B) alkenes
C) alkanes
D) A and B
E) A,B,and C

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
28
The largest known source of tar sands is found in
A) the United States of America.
B) United Arab Emirates.
C) Venezuela.
D) Saudi Arabia.
E) Canada.
A) the United States of America.
B) United Arab Emirates.
C) Venezuela.
D) Saudi Arabia.
E) Canada.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
29
What quantity,in moles,of oxygen is consumed when 369.3 kJ of energy is evolved from the combustion of a mixture of H2(g)and O2(g)?
H2(g)+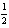 O2(g) H2O( ); rH° = -285.8 kJ/mol-rxn
O2(g) H2O( ); rH° = -285.8 kJ/mol-rxn
A) 0.6461 mol
B) 1.292 mol
C) 0.3869 mol
D) 1.146 mol
E) 0.1461 mol
H2(g)+
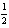 O2(g) H2O( ); rH° = -285.8 kJ/mol-rxn
O2(g) H2O( ); rH° = -285.8 kJ/mol-rxnA) 0.6461 mol
B) 1.292 mol
C) 0.3869 mol
D) 1.146 mol
E) 0.1461 mol

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
30
Methanol reacts with oils and fats to produce biodiesel through a ___ reaction.
A) trans-esterfication
B) addition
C) condensation
D) alkylation
E) substitution
A) trans-esterfication
B) addition
C) condensation
D) alkylation
E) substitution

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
31
The majority of crude oil ends up being used
A) to make synthetic fibers.
B) to make fine chemicals.
C) to make plastics.
D) as fuel.
E) to make rubber.
A) to make synthetic fibers.
B) to make fine chemicals.
C) to make plastics.
D) as fuel.
E) to make rubber.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
32
Rank the three forms of coal,lignite,bituminous,and anthracite,from lowest to highest sulfur content.
A) anthracite < lignite < bituminous
B) lignite < anthracite < bituminous
C) lignite < bituminous < anthracite
D) anthracite < bituminous < lignite
E) bituminous < lignite < anthracite
A) anthracite < lignite < bituminous
B) lignite < anthracite < bituminous
C) lignite < bituminous < anthracite
D) anthracite < bituminous < lignite
E) bituminous < lignite < anthracite

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following statements concerning natural gas is true.
A) Gas fired power plants emit up to 40% less CO2 than coal fired power plants.
B) 70-95% of natural gas consists primarily of ethane (C2H6).
C) Combustion of methane does not produce ash.
D) Large amounts of methane exist in the oceans as methane hydrates.
E) Natural gas is refined before it is transported to distributed for use.
A) Gas fired power plants emit up to 40% less CO2 than coal fired power plants.
B) 70-95% of natural gas consists primarily of ethane (C2H6).
C) Combustion of methane does not produce ash.
D) Large amounts of methane exist in the oceans as methane hydrates.
E) Natural gas is refined before it is transported to distributed for use.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
34
A byproduct of biodiesel production is
A) methanol
B) glycerol
C) water
D) hydrogen
E) glucose
A) methanol
B) glycerol
C) water
D) hydrogen
E) glucose

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
35
In addition to electricity,what products are generated by a fuel cell charged with methanol (CH3OH)and dioxygen (O2)?
A) H2
B) H2 and CO
C) H2 and CH4
D) H2O and CH4
E) H2O and CO2
A) H2
B) H2 and CO
C) H2 and CH4
D) H2O and CH4
E) H2O and CO2

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
36
What process is used to covert coal into coke?
A) steam reforming
B) liquefaction
C) fermentation
D) heating in the absence of air
E) heating in air
A) steam reforming
B) liquefaction
C) fermentation
D) heating in the absence of air
E) heating in air

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following is not a greenhouse gas?
A) CO2
B) CH4
C) H2O
D) N2O
E) N2
A) CO2
B) CH4
C) H2O
D) N2O
E) N2

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
38
Biodiesel is a mixture of
A) fats and oils derived from glycerol.
B) alkenes derived from fats and oils.
C) ketones derived from fatty acids.
D) esters derived from fats and oils.
E) fatty acids derived from fats and oils.
A) fats and oils derived from glycerol.
B) alkenes derived from fats and oils.
C) ketones derived from fatty acids.
D) esters derived from fats and oils.
E) fatty acids derived from fats and oils.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
39
Calculate the mass of coal that produces the same amount of energy when burned as the combustion of 3.53 kg H2.
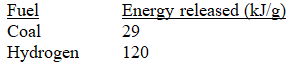
A)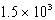 kg
kg
B)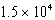 kg
kg
C) 15 kg
D) 0.85 kg
E)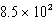 kg
kg
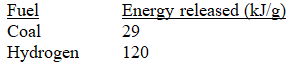
A)
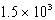 kg
kgB)
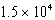 kg
kgC) 15 kg
D) 0.85 kg
E)
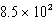 kg
kg
Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
40
At constant pressure and 25 C,what is rH for the following reaction
2C2H6(g)+ 7O2(g) 4CO2(g)+ H2O( )
If the complete consumption of 16.1 g of C2H6 liberates -835.3 kJ of heat energy?
A) -3120 kJ/mol-rxn
B) -1560 kJ/mol-rxn
C) -895 kJ/mol-rxn
D) -447 kJ/mol-rxn
E) -787 kJ/mol-rxn
2C2H6(g)+ 7O2(g) 4CO2(g)+ H2O( )
If the complete consumption of 16.1 g of C2H6 liberates -835.3 kJ of heat energy?
A) -3120 kJ/mol-rxn
B) -1560 kJ/mol-rxn
C) -895 kJ/mol-rxn
D) -447 kJ/mol-rxn
E) -787 kJ/mol-rxn

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
41
What is wrong with the statement,"To stop global worming we must eliminate all greenhouse gases."?

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
42
Explain why the melting of Arctic sea ice will not raise the sea level.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
43
The surface temperature of the Earth has risen in the last 200 years approximately
A) < 0.5 C
B) 1 C
C) 5 C
D) 10 C
E) 15 C
A) < 0.5 C
B) 1 C
C) 5 C
D) 10 C
E) 15 C

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
44
Ocean acidification is the result of the increasing atmospheric concentration of what gas?
A) CH4
B) NO2
C) CO2
D) SO2
E) SO3
A) CH4
B) NO2
C) CO2
D) SO2
E) SO3

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
45
The pH of the oceans in the 18th century was 8. 2.What would the pH be if the hydronium concentration of the oceans was doubled?
A) 16.4
B) 9.28.0
C) 8.0
D) 7.9
E) 4.1
A) 16.4
B) 9.28.0
C) 8.0
D) 7.9
E) 4.1

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
46
Explain why fossil fuels,hydrocarbons produced by natural processes,are considered a nonrenewable energy resource.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
47
Carbonic acid is a diprotic weak acid.Which of the following balanced equilibria represents Ka2 for H2CO3?
A) CO32-(aq)+ H2O( ) OH-(aq)+ HCO3-(aq)
B) CO2(g)+ H2O( ) H2CO3(aq)
C) H2CO3(aq)+ H2O( ) H3O+(aq)+ HCO3-(aq)
D) HCO3-(aq)+ H2O( ) OH-(aq)+ H2CO3(aq)
E) HCO3-(aq)+ H2O( ) H3O+(aq)+ CO32-(aq)
A) CO32-(aq)+ H2O( ) OH-(aq)+ HCO3-(aq)
B) CO2(g)+ H2O( ) H2CO3(aq)
C) H2CO3(aq)+ H2O( ) H3O+(aq)+ HCO3-(aq)
D) HCO3-(aq)+ H2O( ) OH-(aq)+ H2CO3(aq)
E) HCO3-(aq)+ H2O( ) H3O+(aq)+ CO32-(aq)

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
48
One liter of water is mixed with excess CaCO3(s),setting up the following equilibrium.CaCO3(s) Ca2+(aq)+ CO32-(aq)
What is the effect on the solubility of CaCO3(s)as HNO3(aq)is added? (assume no change in volume)
A) the solubility of CaCO3 decreases because the acid increases the amount of CO32-
B) the solubility of CaCO3 increases because the acid increases the amount of CO32-
C) the solubility of CaCO3 decreases because the acid decreases the amount of CO32-
D) the solubility of CaCO3 increases because the acid decreases the amount of CO32-
E) the solubility of CaCO3 is unchanged
What is the effect on the solubility of CaCO3(s)as HNO3(aq)is added? (assume no change in volume)
A) the solubility of CaCO3 decreases because the acid increases the amount of CO32-
B) the solubility of CaCO3 increases because the acid increases the amount of CO32-
C) the solubility of CaCO3 decreases because the acid decreases the amount of CO32-
D) the solubility of CaCO3 increases because the acid decreases the amount of CO32-
E) the solubility of CaCO3 is unchanged

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck



