Deck 24: Carbon: Not Just Another Element
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/93
Play
Full screen (f)
Deck 24: Carbon: Not Just Another Element
1
Which of the following structural formulas contains an error?
A)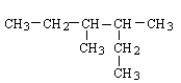
B)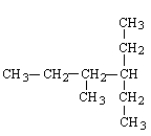
C)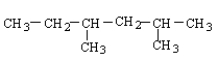
D)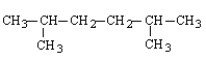
E)
A)
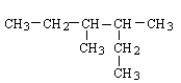
B)

C)
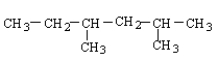
D)
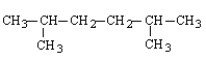
E)


2
Which one of the following statements concerning isomers is INCORRECT?
A) Pairs of nonsuperimposable molecules are called enantiomers.
B) Enantiomers have identical melting and boiling points.
C) Molecules with two or more geometric isomers are termed chiral pairs.
D) Optical isomers rotate polarized light in opposite directions.
E) Structural isomers have the same composition,but the atoms are linked in different ways.
A) Pairs of nonsuperimposable molecules are called enantiomers.
B) Enantiomers have identical melting and boiling points.
C) Molecules with two or more geometric isomers are termed chiral pairs.
D) Optical isomers rotate polarized light in opposite directions.
E) Structural isomers have the same composition,but the atoms are linked in different ways.
Molecules with two or more geometric isomers are termed chiral pairs.
3
Which of the following statements concerning isomers is/are correct?
1)Two types of stereoisomers exist: geometric isomers and optical isomers.
2)Optical isomers are molecules with nonsuperimposable mirror images.
3)Ethanol (CH3CH2OH)and dimethyl ether (CH3OCH3)are examples of geometric isomers.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
1)Two types of stereoisomers exist: geometric isomers and optical isomers.
2)Optical isomers are molecules with nonsuperimposable mirror images.
3)Ethanol (CH3CH2OH)and dimethyl ether (CH3OCH3)are examples of geometric isomers.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
1 and 2
4
Which of the following molecules might be a cycloalkane?
A) C3H4
B) C4H10
C) C5H12
D) C6H10
E) C7H14
A) C3H4
B) C4H10
C) C5H12
D) C6H10
E) C7H14

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following statements concerning structural isomers is/are correct?
1)Structural isomers have the same elemental composition,but the atoms are linked in different ways.
2)Structural isomers have identical physical properties,but different chemical properties.
3)Structural isomers have identical structures,but contain different isotopes of the same elements.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
1)Structural isomers have the same elemental composition,but the atoms are linked in different ways.
2)Structural isomers have identical physical properties,but different chemical properties.
3)Structural isomers have identical structures,but contain different isotopes of the same elements.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
6
What is the molecular formula for heptane?
A) C6H12
B) C6H14
C) C7H14
D) C7H16
E) C9H20
A) C6H12
B) C6H14
C) C7H14
D) C7H16
E) C9H20

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
7
Which one of the following formulas could represent an unsaturated hydrocarbon?
A) C2H6
B) C3H8
C) C4H10
D) C6H12
E) C7H16
A) C2H6
B) C3H8
C) C4H10
D) C6H12
E) C7H16

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
8
The reaction of methane with chlorine in the presence of ultraviolet radiation can produce ____.
A) chloromethane
B) dichloromethane
C) trichloromethane
D) carbon tetrachloride
E) all of these products
A) chloromethane
B) dichloromethane
C) trichloromethane
D) carbon tetrachloride
E) all of these products

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following is the general formula of an alkyne containinng one triple bond?
A) CnH2n+2
B) C2nH2n-2
C) CnHn
D) CnH2n
E) Cn+2Hn
A) CnH2n+2
B) C2nH2n-2
C) CnHn
D) CnH2n
E) Cn+2Hn

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is a molecule?
A) CH3CBr2Cl
B) CBrCl2I
C) CH3CHBrCH2CH3
D) (CH3)2CHCl
E) CH2BrI
A) CH3CBr2Cl
B) CBrCl2I
C) CH3CHBrCH2CH3
D) (CH3)2CHCl
E) CH2BrI

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
11
Four of the following compounds are structural isomers.Which one is
A)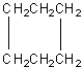
B)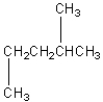
C)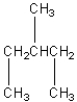
D)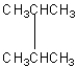
E)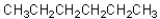
A)

B)

C)

D)

E)
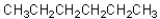

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
12
The carbon-carbon bonding of cyclobutane is shown below.How many hydrogen atoms are needed to complete the structural formula of cyclobutane? 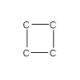
A) 6
B) 8
C) 10
D) 12
E) 14

A) 6
B) 8
C) 10
D) 12
E) 14

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following molecules may be a cycloalkene?
A) C4H8
B) C5H12
C) C5H10
D) C6H12
E) C6H10
A) C4H8
B) C5H12
C) C5H10
D) C6H12
E) C6H10

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following alkenes is capable of forming cis-trans isomers?
A) (CH3)2 = CH2
B) (CH3)2 = CHOH
C) CH3CH = CH2
D) CH3CH = CF2
E) CHBr = CHD
A) (CH3)2 = CH2
B) (CH3)2 = CHOH
C) CH3CH = CH2
D) CH3CH = CF2
E) CHBr = CHD

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
15
What are the two types of stereoisomers?
A) geometrical and optical
B) optical and positional
C) geometrical and positional
D) geometrical and functional group
E) chiral and optical
A) geometrical and optical
B) optical and positional
C) geometrical and positional
D) geometrical and functional group
E) chiral and optical

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following noncyclic hydrocarbons has two bonds?
A) C3H4
B) C4H8
C) C6H12
D) C7H14
E) C8H18
A) C3H4
B) C4H8
C) C6H12
D) C7H14
E) C8H18

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following is a geometric isomer of the structure given below? 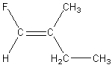
A)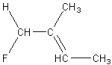
B)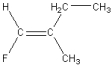
C)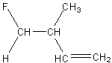
D)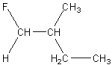
E)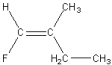

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
18
Which one of the following molecules has a chiral center?
A)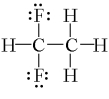
B)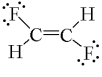
C)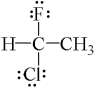
D)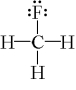
E)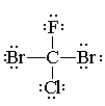
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
19
What is the molecular formula of a cyclic alkane with 7 carbon atoms?
A) C7 H16
B) C7 H12
C) C7 H18
D) C7 H14
E) C7 H7
A) C7 H16
B) C7 H12
C) C7 H18
D) C7 H14
E) C7 H7

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following is an example of a hydrocarbon structural formula?
A) C6H12
B)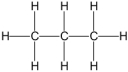
C)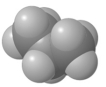
D)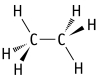
E) CH3CH2CH2CH3
A) C6H12
B)

C)

D)

E) CH3CH2CH2CH3

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
21
What is the name of the following compound? 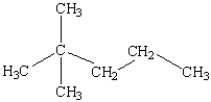
A) 1,1,1-trimethylbutane
B) 3-butylpropane
C) 2-methyl-2-propylpropane
D) 4,4-dimethylpentane
E) 2,2-dimethylpentane

A) 1,1,1-trimethylbutane
B) 3-butylpropane
C) 2-methyl-2-propylpropane
D) 4,4-dimethylpentane
E) 2,2-dimethylpentane

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
22
What is the balanced chemical equation for the combustion of 4-methyl-1-pentene?
A) 2 C5H10(g)+ 15 O2(g) 10 CO2(g)+ 10 H2O(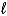 )
)
B) 2 C4H10(g)+ 13 O2(g) 8 CO2(g)+ 10 H2O(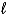 )
)
C) C4H8(g)+ 6 O2(g) 4 CO2(g)+ 4 H2O(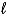 )
)
D) C6H12(g)+ 9 O2(g) 6 CO2(g)+ 6 H2O(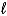 )
)
E) C5H12(g)+ 13 O2(g) 5 CO2 (g)+ 6 H2O(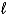 )
)
A) 2 C5H10(g)+ 15 O2(g) 10 CO2(g)+ 10 H2O(
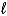 )
)B) 2 C4H10(g)+ 13 O2(g) 8 CO2(g)+ 10 H2O(
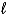 )
)C) C4H8(g)+ 6 O2(g) 4 CO2(g)+ 4 H2O(
 )
)D) C6H12(g)+ 9 O2(g) 6 CO2(g)+ 6 H2O(
 )
)E) C5H12(g)+ 13 O2(g) 5 CO2 (g)+ 6 H2O(
 )
)
Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
23
What is the correct systematic name for the following alkene? 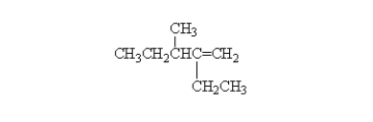
A) 2-ethyl-3-methyl-2-hexene
B) 2-ethyl-3-methyl-1-pentene
C) 4-ethyl-3-methyl-4-pentene
D) 3-methyl-4-ethyl-4-pentene
E) octene

A) 2-ethyl-3-methyl-2-hexene
B) 2-ethyl-3-methyl-1-pentene
C) 4-ethyl-3-methyl-4-pentene
D) 3-methyl-4-ethyl-4-pentene
E) octene

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
24
What type of hydrocarbon is the following molecule? 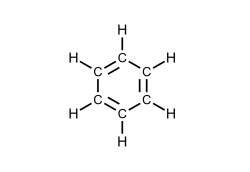
A) alkane
B) alkene
C) alkyne
D) aromatic
E) cycloalkene

A) alkane
B) alkene
C) alkyne
D) aromatic
E) cycloalkene

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
25
Which one of the following hydrocarbons has cis and trans isomers?
A) hexane
B) 1-pentene
C) 2-methylpropane
D) 2-ethylbutane
E) 2-pentene
A) hexane
B) 1-pentene
C) 2-methylpropane
D) 2-ethylbutane
E) 2-pentene

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
26
How many isomers exist for the following benzene derivative,C6H4ClBr?
A) 2
B) 3
C) 4
D) 5
E) none
A) 2
B) 3
C) 4
D) 5
E) none

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
27
Name the following:
CH3-(CH2)5 -CH3
A) heptane
B) pentane
C) butane
D) octane
E) hexane
CH3-(CH2)5 -CH3
A) heptane
B) pentane
C) butane
D) octane
E) hexane

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
28
What is the name of the following compound? 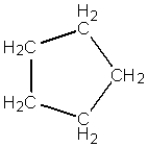
A) pentanol
B) cyclopentane
C) cyclohexane
D) cyclopentene
E) 1,5-pentane

A) pentanol
B) cyclopentane
C) cyclohexane
D) cyclopentene
E) 1,5-pentane

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
29
What is the product of the addition of HCl to ethylene?
A) chloroethane
B) chloroethylene
C) 1,2-dichloroethane
D) 1,1-dichloroethane
E) 1,1,2,2-tetrachloroethylene
A) chloroethane
B) chloroethylene
C) 1,2-dichloroethane
D) 1,1-dichloroethane
E) 1,1,2,2-tetrachloroethylene

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
30
The addition of Br2 is used as the reaction to distinguish between alkanes and alkenes.What is the observation that accompanies this test?
A) A bright red color is produced when Br2 reacts with an alkene.
B) Bromine dissolves in alkanes but not in alkenes.
C) Bromine is a dark red liquid.When it adds to the double bond of an alkene to make the dibromide,it becomes colorless.
D) The red color of bromine disappears when it dissolves in alkanes.
E) Bromine reacts with alkanes to form a precipitate.
A) A bright red color is produced when Br2 reacts with an alkene.
B) Bromine dissolves in alkanes but not in alkenes.
C) Bromine is a dark red liquid.When it adds to the double bond of an alkene to make the dibromide,it becomes colorless.
D) The red color of bromine disappears when it dissolves in alkanes.
E) Bromine reacts with alkanes to form a precipitate.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
31
What is the product of the addition of excess F2 to acetylene?
A) 1,2-difluoroethane
B) ethylene
C) 1,1,2,2-tetrafluoroethane
D) ethane
E) 1,1-difluoroethylene
A) 1,2-difluoroethane
B) ethylene
C) 1,1,2,2-tetrafluoroethane
D) ethane
E) 1,1-difluoroethylene

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
32
What is the structure for 3,4-dimethylhexane?
A)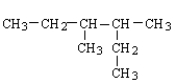
B)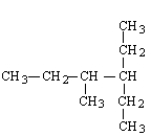
C)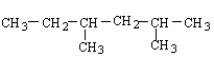
D)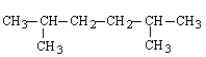
E)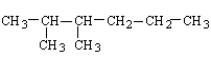
A)
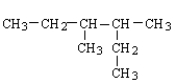
B)

C)
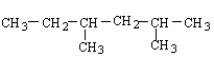
D)
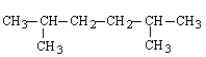
E)
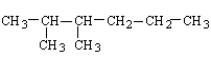

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
33
Name the following: 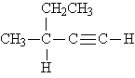
A) 1-hexyne
B) 2-ethynyl butane
C) 2-ethyl-3-butyne
D) 3-methyl-1-pentyne
E) 3-methyl-4-pentyne

A) 1-hexyne
B) 2-ethynyl butane
C) 2-ethyl-3-butyne
D) 3-methyl-1-pentyne
E) 3-methyl-4-pentyne

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
34
An unsaturated hydrocarbon is
A) a hydrocarbon that contains oxygen.
B) a compound in which all carbon atoms have four single bonds.
C) a compound in which one or more carbon atoms have double or triple bonds.
D) a hydrocarbon that is dissolved in water.
E) a cycloalkane with five or more carbons.
A) a hydrocarbon that contains oxygen.
B) a compound in which all carbon atoms have four single bonds.
C) a compound in which one or more carbon atoms have double or triple bonds.
D) a hydrocarbon that is dissolved in water.
E) a cycloalkane with five or more carbons.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
35
What is the major product upon addition of HCl to 1-hexene?
A) CH3CH2CH2CH2CHClCH2Cl
B) CH3CH2CH2CH2CH2CH3
C) CH3CH2CH2CH2CHClCH3
D) CH3CH2CH2CH2CH2CH2Cl
E) CH3CH2CH2CH2CHCl=CH2
A) CH3CH2CH2CH2CHClCH2Cl
B) CH3CH2CH2CH2CH2CH3
C) CH3CH2CH2CH2CHClCH3
D) CH3CH2CH2CH2CH2CH2Cl
E) CH3CH2CH2CH2CHCl=CH2

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
36
What is the name of the following compound? 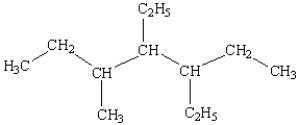
A) 3,4-diethyl-5-methylheptane
B) 3,4,5-heptane
C) 3-ethyl-5-(2-butyl)hexane
D) 3-(2-butyl)-3-ethylhexane
E) 3,5,6-trimethylheptane

A) 3,4-diethyl-5-methylheptane
B) 3,4,5-heptane
C) 3-ethyl-5-(2-butyl)hexane
D) 3-(2-butyl)-3-ethylhexane
E) 3,5,6-trimethylheptane

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
37
What is the expected product from the reaction of 2,3-dimethyl-1-hexene with hydrogen gas?
A) 2,2-dimethylhexane
B) 2,3-dimethylhexane
C) 3,3-dimethylhexane
D) 2-methylheptane
E) 2,3-dimethyl-2-hexene
A) 2,2-dimethylhexane
B) 2,3-dimethylhexane
C) 3,3-dimethylhexane
D) 2-methylheptane
E) 2,3-dimethyl-2-hexene

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
38
What is the name of the following compound? 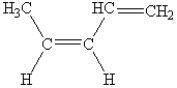
A) trans-acetylene
B) cis-1,3-pentadiene
C) trans-2,4-pentadiene
D) cis-4-methyl-1,3-butadiene
E) cis-4-methyl-1,3-butene

A) trans-acetylene
B) cis-1,3-pentadiene
C) trans-2,4-pentadiene
D) cis-4-methyl-1,3-butadiene
E) cis-4-methyl-1,3-butene

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following is the correct formula for 2-methyl-1-butene?
A)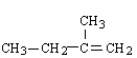
B)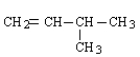
C)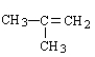
D)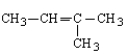
E) none of these
A)
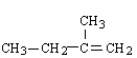
B)
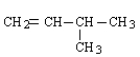
C)
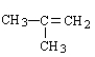
D)
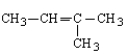
E) none of these

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
40
How many isomers,both structural and geometric,have the molecular formula C6H14?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
41
Which functional group does contain a double bond to an oxygen atom?
A) ester
B) aldehyde
C) ether
D) amide
E) ketone
A) ester
B) aldehyde
C) ether
D) amide
E) ketone

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
42
How many structural isomers possessing the alcohol functional group have the molecular formula C4H10O?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following types of compounds must have an sp2-hybridized carbon center?
A) aldehydes
B) ethers
C) alkynes
D) amines
E) alcohols
A) aldehydes
B) ethers
C) alkynes
D) amines
E) alcohols

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following statements concerning hydrocarbons is/are CORRECT?
1)Coal is a source of many aromatic hydrocarbons.
2)Petroleum is subjected to a process known as cracking,where aromatic rings are cracked open to yield alkane chains.
3)The primary components of petroleum (prior to refining)are alkenes and alkynes.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 1,2,and 3
1)Coal is a source of many aromatic hydrocarbons.
2)Petroleum is subjected to a process known as cracking,where aromatic rings are cracked open to yield alkane chains.
3)The primary components of petroleum (prior to refining)are alkenes and alkynes.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 1,2,and 3

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following alcohols is likely to be least soluble in water?
A) 3-pentanol
B) 2-butanol
C) 1,2-ethanediol
D) 1,2,3-propanetriol
E) methanol
A) 3-pentanol
B) 2-butanol
C) 1,2-ethanediol
D) 1,2,3-propanetriol
E) methanol

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
46
What is the name of the following benzene derivative? 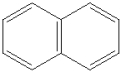
A) dibenzene
B) 1,2-dibenzene
C) toluene
D) naphthalene
E) aniline

A) dibenzene
B) 1,2-dibenzene
C) toluene
D) naphthalene
E) aniline

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following chemical equations depicts a halogenation reaction with benzene?
A) C6H6( )+ 3 Br2(g) 3 C2H2Br2(g)
)+ 3 Br2(g) 3 C2H2Br2(g)
B) C6H6( )+ Cl2(g) C6H5Cl(
)+ Cl2(g) C6H5Cl(  )+ HCl(g)
)+ HCl(g)
C) C6H6( )+ CH3I(g) C6H5CH3(
)+ CH3I(g) C6H5CH3(  )+ HI(g)
)+ HI(g)
D) C6H6( )+ Cl2(g) C6H4Cl2(
)+ Cl2(g) C6H4Cl2(  )+ H2(g)
)+ H2(g)
E) C6H6( )+ 3 Br2(g) 6 C6H6Br6(g)
)+ 3 Br2(g) 6 C6H6Br6(g)
A) C6H6(
 )+ 3 Br2(g) 3 C2H2Br2(g)
)+ 3 Br2(g) 3 C2H2Br2(g)B) C6H6(
 )+ Cl2(g) C6H5Cl(
)+ Cl2(g) C6H5Cl(  )+ HCl(g)
)+ HCl(g)C) C6H6(
 )+ CH3I(g) C6H5CH3(
)+ CH3I(g) C6H5CH3(  )+ HI(g)
)+ HI(g)D) C6H6(
 )+ Cl2(g) C6H4Cl2(
)+ Cl2(g) C6H4Cl2(  )+ H2(g)
)+ H2(g)E) C6H6(
 )+ 3 Br2(g) 6 C6H6Br6(g)
)+ 3 Br2(g) 6 C6H6Br6(g)
Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
48
Formulas for derivatives of hydrocarbons may be written as R-X,where R is a hydrocarbon lacking a hydrogen atom and X is a functional group.Which of the following formulas represents a primary amine?
A) RNH2
B) RCONH2
C) R2NH
D) RCO2R'
E) R3N
A) RNH2
B) RCONH2
C) R2NH
D) RCO2R'
E) R3N

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
49
How many structures (i.e.,unique isomers)exist for tribromobenzene?
A) 2
B) 3
C) 4
D) 5
E) 6
A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
50
Putrescine and cadaverine are two compounds associated with decaying flesh or bad breath.These compounds belong to a class of molecules called ____.
A) ethers
B) amines
C) ketones
D) esters
E) carboxylic acids
A) ethers
B) amines
C) ketones
D) esters
E) carboxylic acids

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following reactions is a substitution reaction?
A) C6H6 + Br2 C6H5Br + HBr
B) CH2=CH2 + Br2 CH2BrCH2Br
C) CH2=CH2 + HBr CH3CH2Br
D) CH2=CH2 + H2 CH3CH3
E) HC CH + 2Br2 CHBr2CHBr2
A) C6H6 + Br2 C6H5Br + HBr
B) CH2=CH2 + Br2 CH2BrCH2Br
C) CH2=CH2 + HBr CH3CH2Br
D) CH2=CH2 + H2 CH3CH3
E) HC CH + 2Br2 CHBr2CHBr2

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following statements concerning methanol is/are CORRECT?
1)Methanol is used as a fuel in high-powered racing cars.
2)Human consumption of methanol can lead to blindness.
3)Methanol is converted by the human body to formaldehyde and formic acid.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 1,2,and 3
1)Methanol is used as a fuel in high-powered racing cars.
2)Human consumption of methanol can lead to blindness.
3)Methanol is converted by the human body to formaldehyde and formic acid.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 1,2,and 3

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
53
Which functional group does contain an oxygen atom?
A) ester
B) ether
C) aldehyde
D) amide
E) amine
A) ester
B) ether
C) aldehyde
D) amide
E) amine

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
54
Which one of the following functional groups is a base?
A) aldehyde
B) alcohol
C) carboxylic acid
D) ether
E) amine
A) aldehyde
B) alcohol
C) carboxylic acid
D) ether
E) amine

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following chemical equations depicts an alkylation reaction?
A) C6H6( )+ CH3Cl(
)+ CH3Cl(  ) C6H5CH3(
) C6H5CH3(  )+ HCl(g)
)+ HCl(g)
B) 2 CH3OH( )+ 3 O2(g) 2 CO2(g)+ 4 H2O(
)+ 3 O2(g) 2 CO2(g)+ 4 H2O(  )
)
C) C6H12( ) C6H10(
) C6H10(  )+ H2(g)
)+ H2(g)
D) CH2ClCH2Cl(g)+ H2(g) CH3CH3(g)+ Cl2(g)
E) CHClCHCl(g) CH2ClCH2Cl(g)
A) C6H6(
 )+ CH3Cl(
)+ CH3Cl(  ) C6H5CH3(
) C6H5CH3(  )+ HCl(g)
)+ HCl(g)B) 2 CH3OH(
 )+ 3 O2(g) 2 CO2(g)+ 4 H2O(
)+ 3 O2(g) 2 CO2(g)+ 4 H2O(  )
)C) C6H12(
 ) C6H10(
) C6H10( 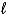 )+ H2(g)
)+ H2(g)D) CH2ClCH2Cl(g)+ H2(g) CH3CH3(g)+ Cl2(g)
E) CHClCHCl(g) CH2ClCH2Cl(g)

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the structures below has the common name m-dichlorobenzene (where m- is meta)?
A)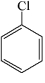
B)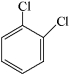
C)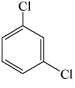
D)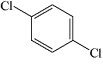
E)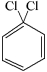
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
57
What is the name of the following benzene derivative? 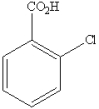
A) 1-chlorobenzoic acid
B) 5-chloroanaline
C) 1-acetate-1-chlorobenzene
D) 5-chlorobenzoic acid
E) 1,3-chlorocarboxylic benzene

A) 1-chlorobenzoic acid
B) 5-chloroanaline
C) 1-acetate-1-chlorobenzene
D) 5-chlorobenzoic acid
E) 1,3-chlorocarboxylic benzene

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
58
Which set of functional groups are present in the molecule shown below? 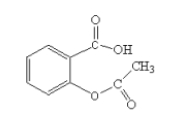
A) aromatic,carboxylic acid,and ester
B) aromatic,alcohol,and ester
C) alkene,alcohol,and ether
D) alkene,carboxylic acid,and ketone
E) aromatic alcohol,ether,and aldehyde

A) aromatic,carboxylic acid,and ester
B) aromatic,alcohol,and ester
C) alkene,alcohol,and ether
D) alkene,carboxylic acid,and ketone
E) aromatic alcohol,ether,and aldehyde

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
59
Identify the product(s)of the hydrogenation of cis-2-hexene.
A) 2-hydroxyhexane
B) 2,3-dihydroxyhexane
C) trans-2-hexane
D) carbon dioxide and water
E) hexane
A) 2-hydroxyhexane
B) 2,3-dihydroxyhexane
C) trans-2-hexane
D) carbon dioxide and water
E) hexane

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
60
Formulas for derivatives of hydrocarbons may be written as R-X,where R is a hydrocarbon lacking a hydrogen atom and X is a functional group.Which of the following formulas represents an ester?
A) ROH
B) ROR'
C) RCOR'
D) RCO2R'
E) RCHO
A) ROH
B) ROR'
C) RCOR'
D) RCO2R'
E) RCHO

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
61
CH3CH2CH2COOH is ____.
A) isobutyl alcohol
B) butyl alcohol
C) propionic acid
D) butanoic acid
E) oxalic acid
A) isobutyl alcohol
B) butyl alcohol
C) propionic acid
D) butanoic acid
E) oxalic acid

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
62
What is the product of the reduction of butanone?
A) 1-butene
B) 2-butanol
C) cis-2-butene
D) acetone
E) butanoic acid
A) 1-butene
B) 2-butanol
C) cis-2-butene
D) acetone
E) butanoic acid

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
63
What are the products of a condensation reaction between a primary amine and a carboxylic acid?
A) water and an amide
B) water and an ester
C) ammonia and an amide
D) ammonia and an ester
E) an ammonium cation and a carboxylate anion
A) water and an amide
B) water and an ester
C) ammonia and an amide
D) ammonia and an ester
E) an ammonium cation and a carboxylate anion

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following statements about polymers is/are correct?
1)Polypropylene and polyethylene are high molecular weight alkenes.
2)Addition polymers must be made from monomers which have at least one double or triple bond somewhere in the molecule.
3)Condensation polymerization reactions produce small molecules,like H2O,as byproducts to the polymer formation.
A) 1 only
B) 2 only
C) 3 only
D) 2 and 3
E) 1,2,and 3
1)Polypropylene and polyethylene are high molecular weight alkenes.
2)Addition polymers must be made from monomers which have at least one double or triple bond somewhere in the molecule.
3)Condensation polymerization reactions produce small molecules,like H2O,as byproducts to the polymer formation.
A) 1 only
B) 2 only
C) 3 only
D) 2 and 3
E) 1,2,and 3

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
65
What is the name of the product of the reaction that occurs when a mixture of methanol and propionic acid is heated in the presence of acid?
A) methyl propylether
B) 2-propanone
C) propyl methanoate
D) 2-methylpropanal
E) methyl propanoate
A) methyl propylether
B) 2-propanone
C) propyl methanoate
D) 2-methylpropanal
E) methyl propanoate

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
66
When a secondary alcohol is oxidized,the product is a(n)____.
A) ketone
B) ether
C) ester
D) aldehyde
E) amide
A) ketone
B) ether
C) ester
D) aldehyde
E) amide

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
67
What is the classification of the molecule below? 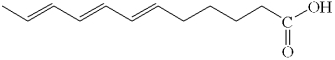
A) polymer
B) fatty acid
C) saccharide
D) carbohydrate
E) triglyceride

A) polymer
B) fatty acid
C) saccharide
D) carbohydrate
E) triglyceride

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
68
When an aldehyde is oxidized,the product is a(n)____.
A) ester
B) ketone
C) ether
D) alcohol
E) carboxylic acid
A) ester
B) ketone
C) ether
D) alcohol
E) carboxylic acid

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
69
The functional group RCONR' is characteristic of a(n)____.
A) ether
B) ester
C) amide
D) aldehyde
E) ketone
A) ether
B) ester
C) amide
D) aldehyde
E) ketone

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following compounds might be used to reduce a carboxylic acid to an aldehyde?
A) H+
B) Cl2
C) K2Cr2O7
D) KMnO4
E) NaBH4
A) H+
B) Cl2
C) K2Cr2O7
D) KMnO4
E) NaBH4

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
71
A mixture of ethanol and benzoic acid is heated in the presence of acid.What is the primary product of the reaction?
A)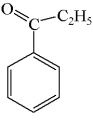
B)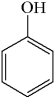
C)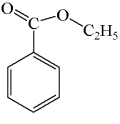
D)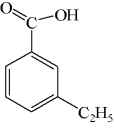
E)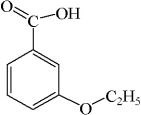
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following statements is/are CORRECT?
1)Thermoplastics are a class of polymers that will not decompose at temperatures as great as 700 C.
2)Thermosetting plastics harden when heated,but they regain their elasticity when cooled.
3)Elastomers are materials that spring back to their original shape when stretched.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
1)Thermoplastics are a class of polymers that will not decompose at temperatures as great as 700 C.
2)Thermosetting plastics harden when heated,but they regain their elasticity when cooled.
3)Elastomers are materials that spring back to their original shape when stretched.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following compounds might be used to oxidize an aldehyde to a carboxylic acid?
A) H2
B) Na
C) LiAlH4
D) KMnO4
E) NaBH4
A) H2
B) Na
C) LiAlH4
D) KMnO4
E) NaBH4

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
74
What class of compounds is responsible for many of the distinctive odors of artificial flavors and perfumes?
A) ethers
B) aldehydes
C) esters
D) amides
E) alkenes
A) ethers
B) aldehydes
C) esters
D) amides
E) alkenes

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
75
A ketone may be produced by oxidation of a(n)_____.
A) alkane
B) alkene
C) secondary alcohol
D) primary alcohol
E) ether
A) alkane
B) alkene
C) secondary alcohol
D) primary alcohol
E) ether

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
76
Reduction of a ketone produces a(n)_____.
A) alkane
B) amide
C) amine
D) secondary alcohol
E) primary alcohol
A) alkane
B) amide
C) amine
D) secondary alcohol
E) primary alcohol

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
77
What is the hybridization and the bond angles around the nitrogen atom in an amide?
A) sp hybridization,180 bond angles
B) sp2 hybridization and 109 bond angles
C) sp2 hybridization and 120 bond angles
D) sp3 hybridization and 109 bond angles
E) sp3 hybridization and 120 bond angles
A) sp hybridization,180 bond angles
B) sp2 hybridization and 109 bond angles
C) sp2 hybridization and 120 bond angles
D) sp3 hybridization and 109 bond angles
E) sp3 hybridization and 120 bond angles

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
78
The reaction of 1-propanol with K2Cr2O7 in acidic solution to produce propanal is what kind of reaction?
A) polymerization
B) combustion
C) oxidation
D) reduction
E) double displacement
A) polymerization
B) combustion
C) oxidation
D) reduction
E) double displacement

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following statements is/are CORRECT?
1)Polyethylene forms by the addition reaction of C2H4 molecules.
2)Copolymers form by the polymerization of two (or more)different monomers.
3)A condensation reaction involves reacting two monomers and splitting out a small molecule,often water.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 1,2,and 3
1)Polyethylene forms by the addition reaction of C2H4 molecules.
2)Copolymers form by the polymerization of two (or more)different monomers.
3)A condensation reaction involves reacting two monomers and splitting out a small molecule,often water.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 1,2,and 3

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
80
The hydrolysis of an ester in the presence of NaOH produces
A) a carboxylate salt of sodium and an alcohol.
B) an ether and an amide.
C) an alcohol and an amine.
D) an aldehyde and an ether.
E) an ether and a carboxylate salt of sodium.
A) a carboxylate salt of sodium and an alcohol.
B) an ether and an amide.
C) an alcohol and an amine.
D) an aldehyde and an ether.
E) an ether and a carboxylate salt of sodium.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck



