Deck 23: The Chemistry of the Transition Elements
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/82
Play
Full screen (f)
Deck 23: The Chemistry of the Transition Elements
1
Which of the following is a transition-metal?
A) vanadium
B) silver
C) iron
D) chromium
E) tin
A) vanadium
B) silver
C) iron
D) chromium
E) tin
tin
2
How many unpaired electrons are found in the ground state electron configuration of cadmium (Cd)?
A) 0
B) 1
C) 2
D) 3
E) 5
A) 0
B) 1
C) 2
D) 3
E) 5
0
3
Which ion produces the red color in rubies?
A) Mn2+
B) Zr4+
C) Pd2+
D) Cr3+
E) Ti4+
A) Mn2+
B) Zr4+
C) Pd2+
D) Cr3+
E) Ti4+
Cr3+
4
Which of the following statements is/are CORRECT?
1)The atomic radii of transition metals decrease initially,then increase as one moves from left to right across a period of the periodic table.
2)The atomic radii of the d-block elements in the 5th and 6th periods in each group are almost identical.
3)The densities of the 6th period transition metals are greater than those of either the 4th or 5th period transition metals.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
1)The atomic radii of transition metals decrease initially,then increase as one moves from left to right across a period of the periodic table.
2)The atomic radii of the d-block elements in the 5th and 6th periods in each group are almost identical.
3)The densities of the 6th period transition metals are greater than those of either the 4th or 5th period transition metals.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
5
Which is the correct valence shell orbital box notation for the ground state electron configuration of Co?
3d 4s
A)
B)
C)
D)
E)
3d 4s
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
6
The white in most white paints comes from ________.
A) TiO2
B) CdS
C) Fe4[Fe(CN)6]3 .14 H2O
D) KMnO4
E) AgI
A) TiO2
B) CdS
C) Fe4[Fe(CN)6]3 .14 H2O
D) KMnO4
E) AgI

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
7
Which element has the following ground state electron configuration?
3d 4s
[Ar]![<strong>Which element has the following ground state electron configuration? ~ ~~~ 3d ~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~ 4s [Ar] </strong> A) Sc B) Mn C) Zn D) Ni E) Fe](https://d2lvgg3v3hfg70.cloudfront.net/TB4499/11ea8937_aba4_c497_a16d_dfd03e73cacc_TB4499_00.jpg)
A) Sc
B) Mn
C) Zn
D) Ni
E) Fe
3d 4s
[Ar]
![<strong>Which element has the following ground state electron configuration? ~ ~~~ 3d ~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~ 4s [Ar] </strong> A) Sc B) Mn C) Zn D) Ni E) Fe](https://d2lvgg3v3hfg70.cloudfront.net/TB4499/11ea8937_aba4_c497_a16d_dfd03e73cacc_TB4499_00.jpg)
A) Sc
B) Mn
C) Zn
D) Ni
E) Fe

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
8
Identify the element with the ground state electron configuration [Xe]4f145d66s2.
A) Ir
B) Ta
C) Os
D) Ru
E) Au
A) Ir
B) Ta
C) Os
D) Ru
E) Au

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following salts would be expected to have unpaired d-electrons?
A) NiCl2
B) CoCl2
C) MnCl2
D) TiCl3
E) ScCl3
A) NiCl2
B) CoCl2
C) MnCl2
D) TiCl3
E) ScCl3

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
10
Which element has the highest density?
A) Zn
B) Y
C) Os
D) Fe
E) Ag
A) Zn
B) Y
C) Os
D) Fe
E) Ag

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
11
The product of the oxidation of iron in most soil in the absence of excess oxygen is black magnetite.The chemical formula of this compound is ____.
A) FeO
B) FeO2
C) Fe2O3
D) Fe3O2
E) Fe3O4
A) FeO
B) FeO2
C) Fe2O3
D) Fe3O2
E) Fe3O4

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
12
Ores are often found mixed with impurities such as sand and clay.In metallurgy,these impurities are called ____.
A) gangue
B) alloys
C) slag
D) roasts
E) precipitates
A) gangue
B) alloys
C) slag
D) roasts
E) precipitates

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following is a characteristic of the transition metals?
A) variable oxidation state
B) compounds that contain their ions are often colored
C) valence electrons in a d subshell
D) high electronegativity
E) the ability to form complex ions
A) variable oxidation state
B) compounds that contain their ions are often colored
C) valence electrons in a d subshell
D) high electronegativity
E) the ability to form complex ions

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
14
If an element can be obtained profitably from a mineral,the mineral is called a(n)____.
A) gangue
B) ore
C) slag
D) roast
E) alloy
A) gangue
B) ore
C) slag
D) roast
E) alloy

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following pairs of d-block metals will have approximately the same atomic radii due to the
A) Fe and Ru
B) Ru and Rh
C) Ru and Os
D) Os and Ir
E) Co and Ni
A) Fe and Ru
B) Ru and Rh
C) Ru and Os
D) Os and Ir
E) Co and Ni

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
16
Which element from the first transition series does NOT react with hydrochloric acid?
A) titanium
B) chromium
C) cobalt
D) nickel
E) copper
A) titanium
B) chromium
C) cobalt
D) nickel
E) copper

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
17
What is the total number of electrons in p orbitals in a ground-state nickel atom?
A) 6
B) 18
C) 12
D) 24
E) 30
A) 6
B) 18
C) 12
D) 24
E) 30

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following ions have an [Ar]3d5 electron configuration: Co2+,Mn2+,and Fe3+?
A) Co2+ only
B) Mn2+ only
C) Fe3+ only
D) Co2+ and Fe3+
E) Mn2+ and Fe3+
A) Co2+ only
B) Mn2+ only
C) Fe3+ only
D) Co2+ and Fe3+
E) Mn2+ and Fe3+

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
19
Iron,_____,and sulfur form the reactive portion of nitrogenase,a biological catalyst used by nitrogen-fixing organisms.
A) Fe
B) Co
C) Zn
D) Cd
E) Mo
A) Fe
B) Co
C) Zn
D) Cd
E) Mo

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
20
What is the maximum oxidation state expected for vanadium?
A) +5
B) +2
C) +3
D) +6
E) +4
A) +5
B) +2
C) +3
D) +6
E) +4

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
21
The only compound containing copper in an ore is chalcopyrite,CuFeS2.If a 90.0 g-sample of this ore contains 22.0 % of copper,what is the percentage of CuFeS2 in the sample?
A) 24.4%
B) 6.86%
C) 0.635%
D) 57.2%
E) 42.8%
A) 24.4%
B) 6.86%
C) 0.635%
D) 57.2%
E) 42.8%

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
22
What two of the following elements or compounds are used to reduce Fe2O3 to Fe in a blast furnace?
A) H2(g)and C(s)
B) Cl2(g)and CO2(g)
C) C(s)and CO(g)
D) CaSiO3(s)and CaO(s)
E) CO2(g)and CaO(s)
A) H2(g)and C(s)
B) Cl2(g)and CO2(g)
C) C(s)and CO(g)
D) CaSiO3(s)and CaO(s)
E) CO2(g)and CaO(s)

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
23
The flexibility,strength,hardness,and malleability of carbon steel is affected by a heating and cooling process called ____.
A) tempering
B) flotation
C) reduction
D) oxidation
E) recrystallization
A) tempering
B) flotation
C) reduction
D) oxidation
E) recrystallization

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
24
The iron produced in a blast furnace contains significant impurities of carbon,phosphorus,and sulfur.These impurities are removed in a basic oxygen furnace,which converts the impurities to ________.
A) CO2,P4O10,and SO2
B) CS2 and P4S3
C) H2CO3,H3PO4,and H2SO3
D) H2CO3,H3PO4,and H2SO4
E) CH4,PH3,and H2S
A) CO2,P4O10,and SO2
B) CS2 and P4S3
C) H2CO3,H3PO4,and H2SO3
D) H2CO3,H3PO4,and H2SO4
E) CH4,PH3,and H2S

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
25
Ions such as [Co(H2O)6]3+ and [Ag(CN)2]- are called ____.
A) ligands
B) Lewis bases
C) chelates
D) alloys
E) coordination complexes
A) ligands
B) Lewis bases
C) chelates
D) alloys
E) coordination complexes

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
26
In which of the following complexes does the transition metal have a d8 configuration?
A) Cu(H2O)62+
B) Fe(CN)63-
C) Ni(CO)4
D) PtCl42-
E) Zn(NH3)42+
A) Cu(H2O)62+
B) Fe(CN)63-
C) Ni(CO)4
D) PtCl42-
E) Zn(NH3)42+

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
27
All of the following metals are generally found in nature as either oxides or sulfides,EXCEPT ____.
A) Fe
B) Au
C) Ni
D) Cr
E) Hg
A) Fe
B) Au
C) Ni
D) Cr
E) Hg

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
28
What is the coordination number of the transition metal ion in [Ag(NH3)2]Cl?
A) 2
B) 3
C) 4
D) 6
E) 8
A) 2
B) 3
C) 4
D) 6
E) 8

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following will not as a ligand to a transition metal cation?
A) O2
B) H3O+
C) PH3
D) NO2-
E) F-
A) O2
B) H3O+
C) PH3
D) NO2-
E) F-

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
30
All of the following metals may be found in nature as free elements EXCEPT ____.
A) Ir
B) Au
C) Pt
D) Ti
E) Rh
A) Ir
B) Au
C) Pt
D) Ti
E) Rh

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
31
In which one of the following is the oxidation state of the underlined metal incorrect?
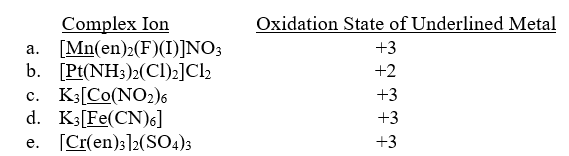

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
32
What is the coordination number of the chromium in [Cr(H2O)2(en)2]3+? The en ligand is pictured below.Not all ligand lone pairs of electrons are explicitly shown. ![<strong>What is the coordination number of the chromium in [Cr(H<sub>2</sub>O)<sub>2</sub>(en)<sub>2</sub>]<sup>3+</sup>? The en ligand is pictured below.Not all ligand lone pairs of electrons are explicitly shown. </strong> A) 2 B) 3 C) 4 D) 5 E) 6](https://d2lvgg3v3hfg70.cloudfront.net/TB4499/11ea8937_aba5_87ee_a16d_73d4df36a7b7_TB4499_00.jpg)
A) 2
B) 3
C) 4
D) 5
E) 6
![<strong>What is the coordination number of the chromium in [Cr(H<sub>2</sub>O)<sub>2</sub>(en)<sub>2</sub>]<sup>3+</sup>? The en ligand is pictured below.Not all ligand lone pairs of electrons are explicitly shown. </strong> A) 2 B) 3 C) 4 D) 5 E) 6](https://d2lvgg3v3hfg70.cloudfront.net/TB4499/11ea8937_aba5_87ee_a16d_73d4df36a7b7_TB4499_00.jpg)
A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
33
Most copper minerals are mined as ________.
A) fluorides
B) sulfides
C) oxides
D) nitrates
E) silicates
A) fluorides
B) sulfides
C) oxides
D) nitrates
E) silicates

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
34
What is the oxidation state of molybdenum in [Mo(H2O)5OH]Cl2?
A) +1
B) +2
C) +3
D) +4
E) +6
A) +1
B) +2
C) +3
D) +4
E) +6

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
35
The calcium silicate formed in the blast furnace reduction of iron ore is called ____.
A) charge
B) gangue
C) pig iron
D) flux
E) slag
A) charge
B) gangue
C) pig iron
D) flux
E) slag

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
36
When 8.1 moles of [Co(NH3)5Cl]Cl2 is dissolved in water,how many moles of ions are in solution?
A) 73
B) 24
C) 5.1
D) 2.7
E) 6.1
A) 73
B) 24
C) 5.1
D) 2.7
E) 6.1

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
37
Copper ores are enriched to increase the percentage of copper by a process called ____.
A) electrolysis
B) flotation
C) roasting
D) tempering
E) filtering
A) electrolysis
B) flotation
C) roasting
D) tempering
E) filtering

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
38
What is the denticity of the following ligand? Not all lone pairs of electrons are explicitly shown. 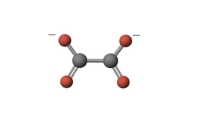
A) 2
B) 3
C) 4
D) 5
E) 6

A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
39
All of the following molecules or ions can act as polydentate ligands EXCEPT ____.
A) phenanthroline; C12H8N2
B) acetylacetate ion; CH3COCHCOCH3-
C) oxalate ion; C2O42-
D) ethylenediamine; H2NCH2CH2NH2
E) dimethylamine; (CH3)2NH2
A) phenanthroline; C12H8N2
B) acetylacetate ion; CH3COCHCOCH3-
C) oxalate ion; C2O42-
D) ethylenediamine; H2NCH2CH2NH2
E) dimethylamine; (CH3)2NH2

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
40
The iron obtained from the blast furnace contains significant impurities of carbon,phosphorus,and sulfur and is called ____.
A) steel
B) pig iron
C) cast iron
D) slag
E) molten iron
A) steel
B) pig iron
C) cast iron
D) slag
E) molten iron

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
41
What is the formula for the pentaamminehydroxoiron(III)ion?
A) [Fe(NH3)5(OH)5]2+
B) [Fe(NH3)5(OH)5]3-
C) [Fe(NH3)5(OH)]2+
D) [Fe(NH3)5(OH)]+
E) [Fe(NH3)(OH)5]3-
A) [Fe(NH3)5(OH)5]2+
B) [Fe(NH3)5(OH)5]3-
C) [Fe(NH3)5(OH)]2+
D) [Fe(NH3)5(OH)]+
E) [Fe(NH3)(OH)5]3-

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following species have geometric isomers: [Fe(en)Cl4]-,[Fe(en)2Cl2]+,and [Fe(en)2BrCl]+?
A) [Fe(en)Cl4]- only
B) [Fe(en)2Cl2]+ only
C) [Fe(en)2BrCl]+ only
D) [Fe(en)2Cl2]+ and [Fe(en)2BrCl]+
E) [Fe(en)Cl4]-,[Fe(en)2Cl2]+,and [Fe(en)2BrCl]+
A) [Fe(en)Cl4]- only
B) [Fe(en)2Cl2]+ only
C) [Fe(en)2BrCl]+ only
D) [Fe(en)2Cl2]+ and [Fe(en)2BrCl]+
E) [Fe(en)Cl4]-,[Fe(en)2Cl2]+,and [Fe(en)2BrCl]+

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
43
What is the formula for potassium diamminetetrachlorovanadate(III)?
A) [KV(NH3)2Cl4]
B) K2[V(NH3)2Cl4]
C) K[V(NH3)2Cl4]
D) [KV(NH3)2]Cl4
E) K[V(NH3)2]Cl4
A) [KV(NH3)2Cl4]
B) K2[V(NH3)2Cl4]
C) K[V(NH3)2Cl4]
D) [KV(NH3)2]Cl4
E) K[V(NH3)2]Cl4

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following pairs of coordination compounds are coordination isomers?
A) [Cr(H2O)6]SO4 and [Cr(NH3)6]SO4
B) [Cr(NH3)5Cl]SO4 and [Cr(NH3)5SO4]Cl
C) [Co(NH3)4(H2O)2]Cl2 and [Co(NH3)2(H2O)4]Cl2
D) [Cr(NH3)6](NO3)2 and [Fe(NH3)6](NO3)3
E) [Cr(H2O)6]Cl2 and [Fe(H2O)6]Cl2
A) [Cr(H2O)6]SO4 and [Cr(NH3)6]SO4
B) [Cr(NH3)5Cl]SO4 and [Cr(NH3)5SO4]Cl
C) [Co(NH3)4(H2O)2]Cl2 and [Co(NH3)2(H2O)4]Cl2
D) [Cr(NH3)6](NO3)2 and [Fe(NH3)6](NO3)3
E) [Cr(H2O)6]Cl2 and [Fe(H2O)6]Cl2

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
45
Which one of the following complex ions has an optical isomer?
A) [Cu(CN)4]2-
B) [Zn(phen)2]2+
C) [Zn(NH3)2en]2+
D) [Co(H2O)4en]2+
E) [Ni(en)3]2+
A) [Cu(CN)4]2-
B) [Zn(phen)2]2+
C) [Zn(NH3)2en]2+
D) [Co(H2O)4en]2+
E) [Ni(en)3]2+

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
46
Below is a list of complex ions and their structures.One pair is incorrectly matched.Which pair?
Complex Ion / Structure
A) [CoCl6]3- / trigonal bipyramidal
B) [Cd(NH3)4]2+ / tetrahedral
C) [NiCl4]2- / tetrahedral
D) [Au(CN)2]- / linear
E) [Co(CN)6]3-/ octahedral
Complex Ion / Structure
A) [CoCl6]3- / trigonal bipyramidal
B) [Cd(NH3)4]2+ / tetrahedral
C) [NiCl4]2- / tetrahedral
D) [Au(CN)2]- / linear
E) [Co(CN)6]3-/ octahedral

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following may act as an ligand,forming coordination isomers known as
A) NH3
B) OH-
C) SO42-
D) CO
E) H2S
A) NH3
B) OH-
C) SO42-
D) CO
E) H2S

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
48
What is the formula for dicyanobis(ethylenediamine)zirconium(IV)nitrate? (en = ethylenediamine)
A) Zr[(CN)2(NO3)2(en)2]
B) [Zr(CN)2(NO3)2(en)2]
C) (NO3)2[Zr(CN)2(en)2]
D) [Zr(CN)2(en)2](NO3)2
E) [Zr(NO3)2(en)2](CN)2
A) Zr[(CN)2(NO3)2(en)2]
B) [Zr(CN)2(NO3)2(en)2]
C) (NO3)2[Zr(CN)2(en)2]
D) [Zr(CN)2(en)2](NO3)2
E) [Zr(NO3)2(en)2](CN)2

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
49
Geometric isomers with a cis or trans configuration are possible for which of the following geometries?
1)octahedral
2)tetrahedral
3)square planar
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 1,2,and 3
1)octahedral
2)tetrahedral
3)square planar
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 1,2,and 3

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
50
What are the possible geometries of a metal complex with a coordination number of 6?
1)square planar
2)tetrahedral
3)octahedral
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
1)square planar
2)tetrahedral
3)octahedral
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
51
How many d electrons are present on the metal ion in the complex ion FeCl63-?
A) 7
B) 2
C) 5
D) 4
E) 3
A) 7
B) 2
C) 5
D) 4
E) 3

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
52
What is the name of the compound having the formula [Cr(en)2(H2O)2]SO4?
A) dihydroxydiethlyenediamminechromate(II)sulfate
B) diaquabis(ethylenediamine)sulfatochromate(IV)
C) bis(ethylenediamine)diaquachromium(II)sulfato
D) diaquabis(ethylenediamine)sulfatochromium(II)
E) diaquabis(ethylenediamine)chromium(II)sulfate
A) dihydroxydiethlyenediamminechromate(II)sulfate
B) diaquabis(ethylenediamine)sulfatochromate(IV)
C) bis(ethylenediamine)diaquachromium(II)sulfato
D) diaquabis(ethylenediamine)sulfatochromium(II)
E) diaquabis(ethylenediamine)chromium(II)sulfate

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
53
What is the correct formula for sodium tetrachlorocuprate(II)?
A) Na3[CuCl4]
B) Na2[CuCl4]
C) Na[CuCl4]
D) Na4[CuCl4]
E) Na2[CuCl6]
A) Na3[CuCl4]
B) Na2[CuCl4]
C) Na[CuCl4]
D) Na4[CuCl4]
E) Na2[CuCl6]

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
54
How many geometric isomers may exist for the square-planar complex ion [Pt(OH)2(NH3)2]2-?
A) 2
B) 3
C) 4
D) 5
E) 6
A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following coordination compounds will immediately form a precipitate when combined with an AgNO3 solution?
A) K[Cr(NH3)2Cl4]
B) K3[Cr(CN)6]
C) [Cr(NH3)6]Cl3
D) [Cr(NH3)2(H2O)Cl3]
E) [Cr(NH3)3Cl3]
A) K[Cr(NH3)2Cl4]
B) K3[Cr(CN)6]
C) [Cr(NH3)6]Cl3
D) [Cr(NH3)2(H2O)Cl3]
E) [Cr(NH3)3Cl3]

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
56
What is a general formula for octahedral complexes that exhibit mer - fac isomerism? (M = metal; A,B and C = ligands)
A) MA5B
B) MA4B2
C) MA3B3
D) MA6
E) MA2B2C2
A) MA5B
B) MA4B2
C) MA3B3
D) MA6
E) MA2B2C2

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
57
What is the name of the compound having the formula K2[PtCl4]?
A) potassium chloroplatinate(II)
B) potassium tetrachloroplatinate(II)
C) potassium chloroplatinate(IV)
D) potassium platanotetrachlorate(II)
E) dipotassium tetrachloroplatnum(II)
A) potassium chloroplatinate(II)
B) potassium tetrachloroplatinate(II)
C) potassium chloroplatinate(IV)
D) potassium platanotetrachlorate(II)
E) dipotassium tetrachloroplatnum(II)

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following compounds may have linkage isomers?
A) [Ni(H2O)4Cl2]Cl
B) [Ni(NH3)4Cl2]NO2
C) [Ni(H2O)5(SCN)]SO4
D) [Ni(H2O)5Cl](SCN)2
E) [Ni(NH3)4Cl2]Cl
A) [Ni(H2O)4Cl2]Cl
B) [Ni(NH3)4Cl2]NO2
C) [Ni(H2O)5(SCN)]SO4
D) [Ni(H2O)5Cl](SCN)2
E) [Ni(NH3)4Cl2]Cl

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
59
Below is a list of formulas for complex compounds; each is matched with its name.One formula / name combination contains an error.Which one?



Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
60
What is the name of the compound having the formula [Ru(CO)6](ClO4)3?
A) hexacarbonmonoxideruthenium(III)perchlorate
B) hexacarbonmonoxideruthenium(II)chloro tetroxide
C) trichloratehexacarbonylruthenate(III)
D) hexacarbonylruthenium(II)chlorate
E) hexacarbonylruthenium(III)perchlorate
A) hexacarbonmonoxideruthenium(III)perchlorate
B) hexacarbonmonoxideruthenium(II)chloro tetroxide
C) trichloratehexacarbonylruthenate(III)
D) hexacarbonylruthenium(II)chlorate
E) hexacarbonylruthenium(III)perchlorate

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
61
What is the number of unpaired electrons in an octahedral,low-spin Mn(II)complex?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
62
Determine the number of unpaired electrons in the tetrahedral complex ion [Co(NH3)4]2+.
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
63
All of the following statements concerning ligand field theory are true EXCEPT
A) in low-spin complexes,electrons are concentrated in the dxy,dyz,and dxz orbitals.
B) in an isolated atom or ion,the five d orbitals have identical energy.
C) low-spin complexes contain the maximum number of unpaired electrons.
D) the crystal field splitting is larger in low-spin complexes than high-spin complexes.
E) the energy difference between d orbitals often corresponds to an energy of visible light.
A) in low-spin complexes,electrons are concentrated in the dxy,dyz,and dxz orbitals.
B) in an isolated atom or ion,the five d orbitals have identical energy.
C) low-spin complexes contain the maximum number of unpaired electrons.
D) the crystal field splitting is larger in low-spin complexes than high-spin complexes.
E) the energy difference between d orbitals often corresponds to an energy of visible light.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
64
Identify the following orbital. 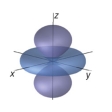
A)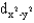
B)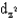
C)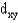
D)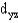
E)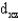

A)
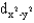
B)
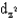
C)
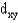
D)
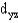
E)
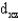

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
65
What is the number of unpaired electrons in an octahedral,high-spin Co(III)complex?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
66
The period 6 transition metals have greater densities than either the period 4 or 5 transition metals.An explanation concerns the 4f orbitals,which are filled with electrons just prior to the 5d orbitals.As the 4f orbitals are filled,the radii of the elements decrease.The decrease in size is referred to as the ________ contraction.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
67
As bound ligands,which of the following causes the largest splitting of d-orbitals?
A) phen
B) H2O
C) I-
D) CN-
E) C2O42-
A) phen
B) H2O
C) I-
D) CN-
E) C2O42-

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
68
Determine the number of unpaired electrons in an octahedral,high-spin Cr(II)complex.
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
69
All of the following complexes will be colored (in the visible)EXCEPT ____.
A) [Co(en)3]2+
B) [Fe(CN)6]4-
C) [Ni(H2O)6]2+
D) [Sc(NH3)6]3+
E) [Cu(NH3)4]2+
A) [Co(en)3]2+
B) [Fe(CN)6]4-
C) [Ni(H2O)6]2+
D) [Sc(NH3)6]3+
E) [Cu(NH3)4]2+

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
70
One method of recovering metals from their ores involves aqueous solutions; a second method involves heat.The method involving heat is called ________.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
71
The spectrochemical series is an arrangement of
A) ligands in order of their tendency to split the d orbitals of a transition metal ion.
B) coordination compounds in order of increasing octahedral ligand field splitting.
C) complex ions in order of the wavelength of light absorbed.
D) ligands in the order of electronegativity.
E) ligands in order of Lewis basicity.
A) ligands in order of their tendency to split the d orbitals of a transition metal ion.
B) coordination compounds in order of increasing octahedral ligand field splitting.
C) complex ions in order of the wavelength of light absorbed.
D) ligands in the order of electronegativity.
E) ligands in order of Lewis basicity.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
72
In an octahedral complex,electrons in 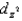 and
and 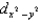
Orbitals experience a greater repulsion from the lone pairs of electrons on ligands because these orbitals
A) contain more electrons than the other three d orbitals.
B) each contain one unpaired electron.
C) are oriented directly toward the incoming ligand electron pairs.
D) are located a greater distance from the metal ion nucleus.
E) are roughly spherical in shape,much like an s orbital.
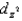 and
and 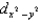
Orbitals experience a greater repulsion from the lone pairs of electrons on ligands because these orbitals
A) contain more electrons than the other three d orbitals.
B) each contain one unpaired electron.
C) are oriented directly toward the incoming ligand electron pairs.
D) are located a greater distance from the metal ion nucleus.
E) are roughly spherical in shape,much like an s orbital.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following d-block ion electron configurations may be high spin low spin when placed in an field?
A) [Ar]3d8
B) [Ar]3d3
C) [Ar]3d9
D) [Ar]3d4
E) [Ar]3d2
A) [Ar]3d8
B) [Ar]3d3
C) [Ar]3d9
D) [Ar]3d4
E) [Ar]3d2

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following cations would be expected to be colorless in aqueous solution?
A) Ni2+
B) Mn2+
C) Co3+
D) Cu2+
E) Ti4+
A) Ni2+
B) Mn2+
C) Co3+
D) Cu2+
E) Ti4+

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following statements concerning octahedral coordination complexes is correct?
A) Strong field ligands produce large octahedral ligand field splittings.
B) Weak field ligands produce high spin complexes.
C) Halide ions are strong field ligands.
D) Weak field ligands result in relatively small values for o.
E) A relatively large value for o causes a complex ion to absorb relatively high energy (shorter wavelength)light.
A) Strong field ligands produce large octahedral ligand field splittings.
B) Weak field ligands produce high spin complexes.
C) Halide ions are strong field ligands.
D) Weak field ligands result in relatively small values for o.
E) A relatively large value for o causes a complex ion to absorb relatively high energy (shorter wavelength)light.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
76
Phenanthroline (C12H8N2)can bind to metal ions using both its ammine groups.It is referred to as a(n)________ ligand,meaning it is "two-toothed."

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
77
The spectrochemical series is
CN- > NO2- > en > NH3 > H2O > OH- > F- >Cl- > Br- >I-
Which of the following complexes will absorb visible light of the longest wavelength?
A) [Co(H2O)6]3+
B) [Co(I)6]3-
C) [Co(OH)6]3-
D) [Co(en)3]3+
E) [Co(NH3)6]3+
CN- > NO2- > en > NH3 > H2O > OH- > F- >Cl- > Br- >I-
Which of the following complexes will absorb visible light of the longest wavelength?
A) [Co(H2O)6]3+
B) [Co(I)6]3-
C) [Co(OH)6]3-
D) [Co(en)3]3+
E) [Co(NH3)6]3+

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
78
Determine the number of unpaired electrons in a Cu(II)tetrahedral complex.
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
79
If an octahedral iron(II)complex is paramagnetic,which of the following sets of conditions best describes the complex?
A) low spin, small
B) low spin, large
C) high spin, small
D) high spin, large
E) none of the above
A) low spin, small
B) low spin, large
C) high spin, small
D) high spin, large
E) none of the above

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following statements is/are CORRECT?
1)Complex ions with the structure [ML2]n are always linear.
2)Complex ions with the structure [ML6]n are always octahedral.
3)Complex ions with the structure [ML4]n are always square planar.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 2 and 3
1)Complex ions with the structure [ML2]n are always linear.
2)Complex ions with the structure [ML6]n are always octahedral.
3)Complex ions with the structure [ML4]n are always square planar.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 2 and 3

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck



