Deck 1: Basic Concepts of Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/53
Play
Full screen (f)
Deck 1: Basic Concepts of Chemistry
1
Use the figures below
A) B)
B)  C)
C) 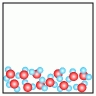 D)
D)  E)
E) 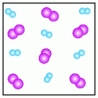
-Which of the above figures represents a ?
A) Figure A
B) Figure B
C) Figure C
D) Figure D
E) Figure E
A)
 B)
B)  C)
C)  D)
D)  E)
E) 
-Which of the above figures represents a ?
A) Figure A
B) Figure B
C) Figure C
D) Figure D
E) Figure E
Figure A
2
Which one of the following is most likely to be a ?
A) campfire smoke
B) blood
C) filtered air
D) soil
E) plain yogurt
A) campfire smoke
B) blood
C) filtered air
D) soil
E) plain yogurt
filtered air
3
Which of the following is a mixture?
A) ice cream
B) gasoline
C) skim milk
D) strawberry jam
E) baking soda (sodium bicarbonate)
A) ice cream
B) gasoline
C) skim milk
D) strawberry jam
E) baking soda (sodium bicarbonate)
baking soda (sodium bicarbonate)
4
A number of the heaviest elements on the periodic table are named for famous scientists.Element number 106 was most likely named for which famous scientist?
A) Alfred Nobel
B) Marie Curie
C) Albert Einstein
D) Glen Seaborg
E) Dmitri Mendeleev
A) Alfred Nobel
B) Marie Curie
C) Albert Einstein
D) Glen Seaborg
E) Dmitri Mendeleev

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
5
Which one of the following is most likely to be a ?
A) table salt dissolved in water
B) soil
C) antifreeze (a mixture of water and ethylene glycol)
D) sugar water
E) gasoline
A) table salt dissolved in water
B) soil
C) antifreeze (a mixture of water and ethylene glycol)
D) sugar water
E) gasoline

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following statements is/are CORRECT?
1)Atoms are the smallest particles of an element that retain the element's chemical properties.
2)Substances composed of only one type of atom are classified as elements.
3)Of the 118 known elements,only 48 occur naturally.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
1)Atoms are the smallest particles of an element that retain the element's chemical properties.
2)Substances composed of only one type of atom are classified as elements.
3)Of the 118 known elements,only 48 occur naturally.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
7
Use the pictures below
A) B)
B)  C)
C)  D)
D)  E)
E) 
-Which of the above figure represents a ?
A) Figure A
B) Figure B
C) Figure C
D) Figure D
E) Figure E
A)
 B)
B)  C)
C)  D)
D)  E)
E) 
-Which of the above figure represents a ?
A) Figure A
B) Figure B
C) Figure C
D) Figure D
E) Figure E

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
8
All of the following statements concerning green chemistry are correct EXCEPT
A) it is better to prevent waste than to treat or clean up waste after it is formed.
B) synthetic methods should be designed to use and generate substances that possess little or no toxicity to human health or the environment.
C) substances used in a chemical process should be chosen to minimize the potential for chemical accidents.
D) raw materials should be renewable whenever technically and economically practical.
E) chemical syntheses should be done at high enough temperatures to ensure harmful bacteria are destroyed.
A) it is better to prevent waste than to treat or clean up waste after it is formed.
B) synthetic methods should be designed to use and generate substances that possess little or no toxicity to human health or the environment.
C) substances used in a chemical process should be chosen to minimize the potential for chemical accidents.
D) raw materials should be renewable whenever technically and economically practical.
E) chemical syntheses should be done at high enough temperatures to ensure harmful bacteria are destroyed.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
9
What is the symbol for the element
A) K
B) Po
C) Pt
D) Kr
E) P
A) K
B) Po
C) Pt
D) Kr
E) P

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
10
Use the pictures below
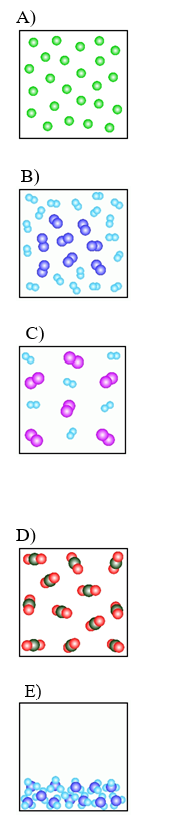
-Which of the above figures represents a ?
A) Figure A
B) Figure B
C) Figure C
D) Figure D
E) Figure E

-Which of the above figures represents a ?
A) Figure A
B) Figure B
C) Figure C
D) Figure D
E) Figure E

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following statements concerning the kinetic-molecular theory of matter is/are CORRECT?
1)Particles in a gas move faster as the temperature increases.
2)Particles in a liquid are packed closely together,but are not confined to specific positions.
3)Particles in a gas vibrate back and forth about an average position.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
1)Particles in a gas move faster as the temperature increases.
2)Particles in a liquid are packed closely together,but are not confined to specific positions.
3)Particles in a gas vibrate back and forth about an average position.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following statements concerning the kinetic-molecular theory of matter is/are CORRECT?
1)Particles in a liquid vibrate back and forth about an average position.
2)Particles in a solid are packed closely together,but are not confined to specific positions.
3)Particles in a gas fly about randomly,colliding with themselves and the walls of their container.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
1)Particles in a liquid vibrate back and forth about an average position.
2)Particles in a solid are packed closely together,but are not confined to specific positions.
3)Particles in a gas fly about randomly,colliding with themselves and the walls of their container.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
13
What is the name of the element with the symbol B?
A) barium
B) beryllium
C) bismuth
D) boron
E) bromine
A) barium
B) beryllium
C) bismuth
D) boron
E) bromine

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
14
What is the correct symbol for potassium?
A) P
B) Pm
C) K
D) Pt
E) Po
A) P
B) Pm
C) K
D) Pt
E) Po

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
15
A is a ________.
A) mathematical formula that models a pattern of behavior
B) set of quantitative data
C) well-tested unifying principle that explains a body of facts
D) tentative explanation or predication based upon experimental observations
E) set of experiments designed to test a theory
A) mathematical formula that models a pattern of behavior
B) set of quantitative data
C) well-tested unifying principle that explains a body of facts
D) tentative explanation or predication based upon experimental observations
E) set of experiments designed to test a theory

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
16
The element whose symbol is Pb is
A) lead.
B) antimony.
C) lanthanum.
D) phosphorus.
E) none of these.
A) lead.
B) antimony.
C) lanthanum.
D) phosphorus.
E) none of these.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following are likely to form a homogeneous mixture?
1) milk and ice cream blended together with chocolate syrup
2) an egg combined with milk and mixed with a whisk
3)1 gram table salt combined with 250 mL of water
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
1) milk and ice cream blended together with chocolate syrup
2) an egg combined with milk and mixed with a whisk
3)1 gram table salt combined with 250 mL of water
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
18
What is the correct symbol for silver?
A) S
B) Si
C) Ag
D) Sr
E) Au
A) S
B) Si
C) Ag
D) Sr
E) Au

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
19
Which one of the following statements is
A) A pure substance may be separated by filtration or distillation into two or more components.
B) A heterogeneous mixture is also known as a solution.
C) A heterogeneous mixture is composed of two or more substances in the same phase.
D) The composition is uniform throughout a homogeneous mixture.
E) The combination of a liquid and a solid always results in a heterogeneous mixture.
A) A pure substance may be separated by filtration or distillation into two or more components.
B) A heterogeneous mixture is also known as a solution.
C) A heterogeneous mixture is composed of two or more substances in the same phase.
D) The composition is uniform throughout a homogeneous mixture.
E) The combination of a liquid and a solid always results in a heterogeneous mixture.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
20
One of the following is a description of Which one is it?
A) easily compressed
B) particles in definite positions
C) relatively low densities
D) fills any container completely
E) expands infinitely on heating
A) easily compressed
B) particles in definite positions
C) relatively low densities
D) fills any container completely
E) expands infinitely on heating

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
21
A pure substance composed of two or more different elements is a(n)________.
A) ion
B) heterogeneous mixture
C) chemical compound
D) solid
E) solution
A) ion
B) heterogeneous mixture
C) chemical compound
D) solid
E) solution

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following is a correct name-symbol combination?
A) vanadium,V
B) silicon,Si
C) aluminum,Al
D) copper,Cu
E) potassium,Ge
A) vanadium,V
B) silicon,Si
C) aluminum,Al
D) copper,Cu
E) potassium,Ge

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following observations is/are examples of physical change?
1)The density of water decreases when it solidifies.
2)Aluminum melts when heated above 660 C.
3)Hydrogen peroxide (H2O2)decomposes to water and oxygen.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
1)The density of water decreases when it solidifies.
2)Aluminum melts when heated above 660 C.
3)Hydrogen peroxide (H2O2)decomposes to water and oxygen.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following statements concerning water (H2O) is/are CORRECT?
1) H2O is a chemical compound.
2) Water is a homogeneous mixture.
3) Liquid water is a mixture of elemental hydrogen and oxygen.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
1) H2O is a chemical compound.
2) Water is a homogeneous mixture.
3) Liquid water is a mixture of elemental hydrogen and oxygen.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
25
Which one of the following statements is not a comparison of physical properties?
A) Potassium reacts with water more quickly than calcium reacts with water.
B) The electrical conductivity of aluminum is greater than copper.
C) The density of copper is less than the density of lead.
D) The solubility of NaCl in hot water is greater than the solubility in cold water.
E) The boiling point of water is greater than the boiling point of ethanol.
A) Potassium reacts with water more quickly than calcium reacts with water.
B) The electrical conductivity of aluminum is greater than copper.
C) The density of copper is less than the density of lead.
D) The solubility of NaCl in hot water is greater than the solubility in cold water.
E) The boiling point of water is greater than the boiling point of ethanol.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
26
Which one of the following statements is not a comparison of physical properties?
A) Mercury and gallium are both liquids at 50 C.
B) Oxygen is more soluble in water than helium.
C) Silver and gold are malleable metals.
D) Oxygen and nitrogen are both liquids at -200 C.
E) Calcium dissolves more quickly than iron in acids.
A) Mercury and gallium are both liquids at 50 C.
B) Oxygen is more soluble in water than helium.
C) Silver and gold are malleable metals.
D) Oxygen and nitrogen are both liquids at -200 C.
E) Calcium dissolves more quickly than iron in acids.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
27
All of the following are examples of intensive properties of matter EXCEPT _______.
A) ductility
B) melting point
C) thermal conductivity
D) weight
E) malleability
A) ductility
B) melting point
C) thermal conductivity
D) weight
E) malleability

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
28
An electrically charged atom or group of atoms is a(n)________.
A) element
B) ion
C) molecule
D) heterogeneous mixture
E) solution
A) element
B) ion
C) molecule
D) heterogeneous mixture
E) solution

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following observations is/are examples of chemical change?
1)Iron (Fe)rusts,forming Fe2O3.
2)The density of water increases when it changes from a solid to a liquid.
3)Sodium chloride melts at 801 C.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 2 and 3
1)Iron (Fe)rusts,forming Fe2O3.
2)The density of water increases when it changes from a solid to a liquid.
3)Sodium chloride melts at 801 C.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 2 and 3

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
30
An intensive property of a substance is
A) independent of the amount present.
B) dependent on its volume,but not its mass.
C) not affected by its temperature.
D) dependent only on its temperature.
E) dependent only on its mass and volume.
A) independent of the amount present.
B) dependent on its volume,but not its mass.
C) not affected by its temperature.
D) dependent only on its temperature.
E) dependent only on its mass and volume.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
31
Which of following would be classified as a change?
A) the condensation of nitrogen gas
B) the dissolution of sugar in water
C) the melting of gold
D) the tarnishing of the surface of copper in the presence of oxygen
E) the transformation of solid carbon dioxide into gaseous carbon dioxide
A) the condensation of nitrogen gas
B) the dissolution of sugar in water
C) the melting of gold
D) the tarnishing of the surface of copper in the presence of oxygen
E) the transformation of solid carbon dioxide into gaseous carbon dioxide

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
32
What is the name of the element with the symbol Cr?
A) cerium
B) carbon
C) chromium
D) cadmium
E) chlorine
A) cerium
B) carbon
C) chromium
D) cadmium
E) chlorine

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
33
Which one of the following substances is classified as a chemical compound?
A) Mo
B) Cr
C) Cd
D) CO
E) No
A) Mo
B) Cr
C) Cd
D) CO
E) No

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
34
What kind of change is depicted below? 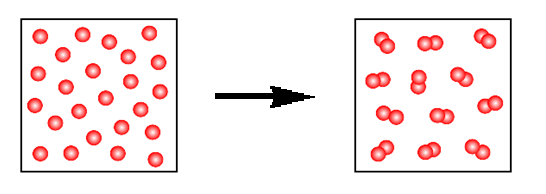
A) both chemical and physical change
B) chemical change
C) no change
D) physical change

A) both chemical and physical change
B) chemical change
C) no change
D) physical change

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following are extensive properties: mass,volume,and/or density?
A) mass only
B) volume only
C) density only
D) mass and volume
E) volume and density
A) mass only
B) volume only
C) density only
D) mass and volume
E) volume and density

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
36
A(n)________ is a pure substance that is composed of only one type of atom.
A) ion
B) solution
C) element
D) molecule
E) gas
A) ion
B) solution
C) element
D) molecule
E) gas

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
37
Which one of the following substances is classified as an element?
A) I2
B) NO
C) KCl
D) C6H12O6
E) CO
A) I2
B) NO
C) KCl
D) C6H12O6
E) CO

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
38
Which term best describes methane,CH4?
A) homogeneous mixture
B) ion
C) element
D) chemical compound
E) atom
A) homogeneous mixture
B) ion
C) element
D) chemical compound
E) atom

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following statements is/are CORRECT?
1)The conduction of electricity through copper wire is a chemical change.
2)The rusting of iron is a chemical change.
3)The evaporation of ammonia at -33.3 C is a chemical change.
A) 1 only
B) 2 only
C) 3 only
D) 2 and 3
E) 1,2,and 3
1)The conduction of electricity through copper wire is a chemical change.
2)The rusting of iron is a chemical change.
3)The evaporation of ammonia at -33.3 C is a chemical change.
A) 1 only
B) 2 only
C) 3 only
D) 2 and 3
E) 1,2,and 3

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
40
A battery-operated power tool,such as a cordless drill,converts
A) electrostatic energy to chemical potential energy.
B) mechanical energy to electrostatic energy.
C) thermal energy to mechanical energy.
D) thermal energy to gravitational energy.
E) chemical potential energy to mechanical energy.
A) electrostatic energy to chemical potential energy.
B) mechanical energy to electrostatic energy.
C) thermal energy to mechanical energy.
D) thermal energy to gravitational energy.
E) chemical potential energy to mechanical energy.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
41
________ energy is the energy associated with the separation of two electrical charges.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
42
To ensure integrity in science,experimental results should be ________ and reported in sufficient detail that the experiment can be repeated by others.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
43
Many regulators,environmentalists,and citizens around the world believe that ________ development is required to meet today's economic and environmental needs while preserving the options for future generations to meet theirs.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following lists contains only forms of kinetic energy?
A) electrostatic,gravitational,and mechanical energy
B) gravitational,mechanical,and electrical energy
C) thermal,acoustic,and mechanical energy
D) chemical,thermal,and acoustic energy
E) gravitational,chemical,and electrostatic energy
A) electrostatic,gravitational,and mechanical energy
B) gravitational,mechanical,and electrical energy
C) thermal,acoustic,and mechanical energy
D) chemical,thermal,and acoustic energy
E) gravitational,chemical,and electrostatic energy

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
45
Of the following types of energy,which is/are classified as potential energy?
1) mechanical
2) electrostatic
3) chemical
A) 1 only
B) 2 only
C) 3 only
D) 2 and 3
E) 1,2,and 3
1) mechanical
2) electrostatic
3) chemical
A) 1 only
B) 2 only
C) 3 only
D) 2 and 3
E) 1,2,and 3

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
46
The ________ of a substance is defined as its mass per unit volume.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
47
Substances like hydrogen (H2) and oxygen (O2) that are composed of only one type of atom are classified as ________.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
48
The law of ________ states that the total energy of the universe is constant.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
49
Properties,such as color and density,which can be observed or measured without changing the composition of a substance are called ________ properties.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
50
A(n)________ is the smallest particle of an element that retains the characteristic chemical properties of that element.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
51
Potential energy possessed by water at the top of a waterfall is known as ________ energy.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
52
A mass of 10 g of table salt dissolves in water to form a(n)________ mixture (i.e.,a mixture that is uniform throughout).

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
53
Density is an example of a(n)________ property,which is one that does not depend on the amount of a substance.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck



