Deck 4: Chemical Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/85
Play
Full screen (f)
Deck 4: Chemical Reactions
1
Which one of the following equations is properly balanced?
A) Sn + 4HNO3 SnO2 + 4NO2 + 2H2O
B) 2Na2SO4 + 3Bi(NO3)3 Bi2(SO4)3 + 9NaNO3
C) CH3CHO + 3O2 2CO2 + 2H2O
D) NH4NO3 2H2O + N2
E) Na2CO3 + 2H2SO4 Na2SO4 + 2H2O + CO2
A) Sn + 4HNO3 SnO2 + 4NO2 + 2H2O
B) 2Na2SO4 + 3Bi(NO3)3 Bi2(SO4)3 + 9NaNO3
C) CH3CHO + 3O2 2CO2 + 2H2O
D) NH4NO3 2H2O + N2
E) Na2CO3 + 2H2SO4 Na2SO4 + 2H2O + CO2
Sn + 4HNO3 SnO2 + 4NO2 + 2H2O
2
Which of the following equations is balanced?
A) 2Sb2OS2 + 10O2 2Sb2O5 + 4SO3
B) (NH4)2Cr2O7 N2 + 4H2O + Cr2O3
C) C12H22O11 + 12O2 12CO2 + 11H2O
D) 2NaCl + Pb(NO3)2 PbCl2 + 2NaNO3
E) Fe3O4 + 3CO 3Fe + 3CO2
A) 2Sb2OS2 + 10O2 2Sb2O5 + 4SO3
B) (NH4)2Cr2O7 N2 + 4H2O + Cr2O3
C) C12H22O11 + 12O2 12CO2 + 11H2O
D) 2NaCl + Pb(NO3)2 PbCl2 + 2NaNO3
E) Fe3O4 + 3CO 3Fe + 3CO2
Fe3O4 + 3CO 3Fe + 3CO2
3
The reaction of elemental chlorine with potassium iodide yields elemental iodine and potassium chloride.Write a balanced chemical equation for this reaction.
A) Cl2(g)+ KI(s) I(s)+ KCl2(s)
B) Cl2(g)+ 2 KI(s) I2(s)+ 2 KCl(s)
C) Cl2(g)+ KI2(s) I2(s)+ KCl2(s)
D) Cl(g)+ KI(s) I(s)+ KCl(s)
E) Cl2(g)+ 2 K2I(s) I2(s)+ 2 K2Cl(s)
A) Cl2(g)+ KI(s) I(s)+ KCl2(s)
B) Cl2(g)+ 2 KI(s) I2(s)+ 2 KCl(s)
C) Cl2(g)+ KI2(s) I2(s)+ KCl2(s)
D) Cl(g)+ KI(s) I(s)+ KCl(s)
E) Cl2(g)+ 2 K2I(s) I2(s)+ 2 K2Cl(s)
Cl2(g)+ 2 KI(s) I2(s)+ 2 KCl(s)
4
What is the smallest whole number coefficient for sodium hydroxide when the following equation is balanced?
__Na2SO4 + __Al(OH)3 __Al2(SO4)3 + __NaOH
A) 1
B) 2
C) 3
D) 4
E) 6
__Na2SO4 + __Al(OH)3 __Al2(SO4)3 + __NaOH
A) 1
B) 2
C) 3
D) 4
E) 6

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following statements is/are CORRECT?
1)Most ionic compounds containing phosphate ion are insoluble in water.
2)Most ionic compounds containing potassium ion are insoluble in water.
3)Most ionic compounds containing hydroxide ion are soluble in water.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
1)Most ionic compounds containing phosphate ion are insoluble in water.
2)Most ionic compounds containing potassium ion are insoluble in water.
3)Most ionic compounds containing hydroxide ion are soluble in water.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
6
The complete combustion of nonane,C9H20,yields carbon dioxide and water: 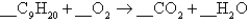 The coefficient of in the balanced equation is
The coefficient of in the balanced equation is
A) 15.
B) 12.
C) 16.
D) 13.
E) 14.
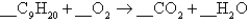 The coefficient of in the balanced equation is
The coefficient of in the balanced equation isA) 15.
B) 12.
C) 16.
D) 13.
E) 14.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
7
A precipitate is expected when an aqueous solution of potassium iodide is added to an aqueous solution of
A) calcium nitrate.
B) barium hydroxide.
C) lead perchlorate.
D) iron(II)chloride.
E) sodium sulfate.
A) calcium nitrate.
B) barium hydroxide.
C) lead perchlorate.
D) iron(II)chloride.
E) sodium sulfate.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following compounds is in water?
A) Sc2O3
B) Cs3N
C) FeS
D) Hg2Br2
E) CdS
A) Sc2O3
B) Cs3N
C) FeS
D) Hg2Br2
E) CdS

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
9
Nitroglycerin decomposes violently according to the balanced chemical equation below.2 C3H5(NO3)3( 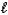 ) 3 N2(g)+ 1/2 O2(g)+ 6 CO2(g)+ 5 H2O(g)
) 3 N2(g)+ 1/2 O2(g)+ 6 CO2(g)+ 5 H2O(g)
Which of the following statements concerning this reaction is/are CORRECT?
1)Two moles of nitroglycerine will produce three moles of nitrogen and five moles of water.
2)Four molecules of nitroglycerine will produced one molecule of oxygen and twelve molecules of carbon dioxide.
3)Six grams of nitroglycerine will produce nine grams of nitrogen and fifteen grams of water.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
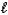 ) 3 N2(g)+ 1/2 O2(g)+ 6 CO2(g)+ 5 H2O(g)
) 3 N2(g)+ 1/2 O2(g)+ 6 CO2(g)+ 5 H2O(g)Which of the following statements concerning this reaction is/are CORRECT?
1)Two moles of nitroglycerine will produce three moles of nitrogen and five moles of water.
2)Four molecules of nitroglycerine will produced one molecule of oxygen and twelve molecules of carbon dioxide.
3)Six grams of nitroglycerine will produce nine grams of nitrogen and fifteen grams of water.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
10
Which one of the statements below is concerning the following reaction:
NH3(g)+ H2O NH4+(aq)+ OH-(aq)
NH4+(aq)+ OH-(aq)
A) The double arrows indicate that ammonia,NH3,is only very slightly soluble in water.
B) The reaction is reversible.
C) When NH3 is added to H2O,NH4+ and OH- ions are produced in a 1:1 ratio.
D) When solutions of NH4+ and OH- are mixed,some ammonia is produced.
E) Ammonia partially reacts with water.
NH3(g)+ H2O
 NH4+(aq)+ OH-(aq)
NH4+(aq)+ OH-(aq)A) The double arrows indicate that ammonia,NH3,is only very slightly soluble in water.
B) The reaction is reversible.
C) When NH3 is added to H2O,NH4+ and OH- ions are produced in a 1:1 ratio.
D) When solutions of NH4+ and OH- are mixed,some ammonia is produced.
E) Ammonia partially reacts with water.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
11
Metals react with oxygen gas to produce oxides with the general formula MxOy.Write a balanced chemical equation for the reaction of titanium with oxygen to yield titanium(IV)oxide.
A) 4 Ti(s)+ O2(g) 2 Ti2O(s)
B) Ti(s)+ O2(g) TiO2(s)
C) 2 Ti(s)+ O2(g) 2 TiO(s)
D) Ti(s)+ O(g) TiO(s)
E) 8 Ti(s)+ O2(g) 2 Ti4O(s)
A) 4 Ti(s)+ O2(g) 2 Ti2O(s)
B) Ti(s)+ O2(g) TiO2(s)
C) 2 Ti(s)+ O2(g) 2 TiO(s)
D) Ti(s)+ O(g) TiO(s)
E) 8 Ti(s)+ O2(g) 2 Ti4O(s)

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following statements is/are CORRECT?
1)Water soluble ionic compounds,such as NaCl,are strong electrolytes.
2)Some molecular compounds,such as HCl,are strong electrolytes.
3)Some molecular compounds,such as acetic acid,are weak electrolytes.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
1)Water soluble ionic compounds,such as NaCl,are strong electrolytes.
2)Some molecular compounds,such as HCl,are strong electrolytes.
3)Some molecular compounds,such as acetic acid,are weak electrolytes.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following statements concerning electrolytes and nonelectrolytes is/are true?
1)Some molecular substances are electrolytes.
2)All electrolytes are ionic substances.
3)Strong electrolytes partially ionize in solution.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 2 and 3
1)Some molecular substances are electrolytes.
2)All electrolytes are ionic substances.
3)Strong electrolytes partially ionize in solution.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 2 and 3

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
14
What is the balanced chemical equation for the complete combustion of benzoic acid,C6H5CO2H,to form carbon dioxide and water?
A) C6H5CO2H(s) 6 C(s)+ CO2(g)+ 3 H2(g)
B) C6H5CO2H(s) 7 CO2(g)+ 3 H2O(g)
C) C6H5CO2H(s)+ O2(g) CO2(g)+ H2O(g)
D) C6H5CO2H(s)+ 8 O2(g) 7 CO2(g)+ 3 H2O(g)
E) 2 C6H5CO2H(s)+ 15 O2(g) 14 CO2(g)+ 6 H2O(g)
A) C6H5CO2H(s) 6 C(s)+ CO2(g)+ 3 H2(g)
B) C6H5CO2H(s) 7 CO2(g)+ 3 H2O(g)
C) C6H5CO2H(s)+ O2(g) CO2(g)+ H2O(g)
D) C6H5CO2H(s)+ 8 O2(g) 7 CO2(g)+ 3 H2O(g)
E) 2 C6H5CO2H(s)+ 15 O2(g) 14 CO2(g)+ 6 H2O(g)

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following statements is/are CORRECT?
1)A solution is a homogeneous mixture of two or more substances.
2)A solute is a mixture of a solvent and a soluble compound.
3)Aqueous solutions are solutions in which water is a solvent.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1 and 3
1)A solution is a homogeneous mixture of two or more substances.
2)A solute is a mixture of a solvent and a soluble compound.
3)Aqueous solutions are solutions in which water is a solvent.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1 and 3

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
16
What are the smallest whole number coefficients the reactants and products when the following molecular equation is correctly balanced?
__NH3(g)+__O2(g) __NO(g)+ __H2O(g)
A) 1,1,1,1
B) 2,3,2,3
C) 3,2,3,4
D) 3,4,3,4
E) 4,5,4,6
__NH3(g)+__O2(g) __NO(g)+ __H2O(g)
A) 1,1,1,1
B) 2,3,2,3
C) 3,2,3,4
D) 3,4,3,4
E) 4,5,4,6

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
17
Which one of the following compounds is a nonelectrolyte when dissolved in water?
A) HCl
B) CaCl2
C) CCl4
D) Cu(NO3)2
E) NaCH3CO2
A) HCl
B) CaCl2
C) CCl4
D) Cu(NO3)2
E) NaCH3CO2

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
18
The products of the complete combustion of octane,C8H18,are carbon dioxide and water.Write a balanced chemical equation for this reaction.
A) C8H18(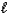 ) 8 C(s)+ 9 H2(g)
) 8 C(s)+ 9 H2(g)
B) C8H18(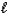 )+ 25 O2(g) 8 CO2(g)+ 9 H2O(g)
)+ 25 O2(g) 8 CO2(g)+ 9 H2O(g)
C) 2 C8H18( )+ 25 O2(g) 16 CO2(g)+ 18 H2O(g)
)+ 25 O2(g) 16 CO2(g)+ 18 H2O(g)
D) C8H18( )+ 16 O2(g) 8 CO2(g)+ 9 H2(g)
)+ 16 O2(g) 8 CO2(g)+ 9 H2(g)
E) 2 C8H18( )+ 17 O2(g) 16 CO(g)+ 18 H2O(g)
)+ 17 O2(g) 16 CO(g)+ 18 H2O(g)
A) C8H18(
 ) 8 C(s)+ 9 H2(g)
) 8 C(s)+ 9 H2(g)B) C8H18(
 )+ 25 O2(g) 8 CO2(g)+ 9 H2O(g)
)+ 25 O2(g) 8 CO2(g)+ 9 H2O(g)C) 2 C8H18(
 )+ 25 O2(g) 16 CO2(g)+ 18 H2O(g)
)+ 25 O2(g) 16 CO2(g)+ 18 H2O(g)D) C8H18(
 )+ 16 O2(g) 8 CO2(g)+ 9 H2(g)
)+ 16 O2(g) 8 CO2(g)+ 9 H2(g)E) 2 C8H18(
 )+ 17 O2(g) 16 CO(g)+ 18 H2O(g)
)+ 17 O2(g) 16 CO(g)+ 18 H2O(g)
Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
19
The balanced chemical equation for the combustion of methane is:
CH4(g)+ 2 O2(g) CO2(g)+ 2 H2O(g)
Which of the following statements concerning this chemical equation is/are correct?
1)One gram of methane gas reacts with two grams of dioxygen gas,producing one gram of carbon dioxide gas and two grams of gaseous water.
2)One mole of methane gas reacts with two moles of dioxygen gas,producing one mole of carbon dioxide gas and two moles of gaseous water.
3)One molecule of methane gas reacts with two molecules of dioxygen gas,producing one molecule of carbon dioxide gas and two molecules of gaseous water.
A) 1 only
B) 2 only
C) 2 and 3
D) 1 and 3
E) 1,2 and 3
CH4(g)+ 2 O2(g) CO2(g)+ 2 H2O(g)
Which of the following statements concerning this chemical equation is/are correct?
1)One gram of methane gas reacts with two grams of dioxygen gas,producing one gram of carbon dioxide gas and two grams of gaseous water.
2)One mole of methane gas reacts with two moles of dioxygen gas,producing one mole of carbon dioxide gas and two moles of gaseous water.
3)One molecule of methane gas reacts with two molecules of dioxygen gas,producing one molecule of carbon dioxide gas and two molecules of gaseous water.
A) 1 only
B) 2 only
C) 2 and 3
D) 1 and 3
E) 1,2 and 3

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following statements is/are CORRECT?
1)Most ionic compounds containing nitrate ion are soluble in water.
2)Most ionic compounds containing sulfate ion are insoluble in water.
3)Most ionic compounds containing carbonate ion are soluble in water.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
1)Most ionic compounds containing nitrate ion are soluble in water.
2)Most ionic compounds containing sulfate ion are insoluble in water.
3)Most ionic compounds containing carbonate ion are soluble in water.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
21
When aqueous solutions of calcium iodide,CaI2,and silver nitrate,AgNO3,are combined,which of the following statements below describes what occurs:
A) A precipitate of Ca(NO3)2 forms
B) A precipitate of AgI forms
C) Both Ca(NO3)2 and AgI precipitate
D) No precipitate will form
A) A precipitate of Ca(NO3)2 forms
B) A precipitate of AgI forms
C) Both Ca(NO3)2 and AgI precipitate
D) No precipitate will form

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
22
What products result from mixing aqueous solutions of Ni(NO3)2(aq)and NaOH(aq)?
A) Ni(OH)2(s),Na+(aq),and NO3-(aq)
B) Ni(OH)2(s)and NaNO3(s)
C) Ni2(OH)2(aq)and NaNO3(aq)
D) Ni(OH)2(aq)and NaNO3(s)
E) Ni(OH)2(s),N2(g),and H2O(l)
A) Ni(OH)2(s),Na+(aq),and NO3-(aq)
B) Ni(OH)2(s)and NaNO3(s)
C) Ni2(OH)2(aq)and NaNO3(aq)
D) Ni(OH)2(aq)and NaNO3(s)
E) Ni(OH)2(s),N2(g),and H2O(l)

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following compounds is in water?
A) NH4Br
B) KBr
C) ZnCl2
D) AgBr
E) NaBr
A) NH4Br
B) KBr
C) ZnCl2
D) AgBr
E) NaBr

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
24
Write a balanced chemical equation for the reaction of aqueous solutions of magnesium chloride and potassium phosphate.
A) MgCl2(aq)+ K3PO4(aq) K3Mg(s)+ PO4Cl2(aq)
B) 3 MgCl2(aq)+ 2 K3PO4(aq) 3 K2Mg(s)+ 2 PO4Cl3(aq)
C) MgCl(aq)+ KPO4(aq) MgPO4(s)+ KCl(aq)
D) MgCl2(aq)+ 2 KPO4(aq) Mg(PO4)2(s)+ 2 KCl(aq)
E) 3 MgCl2(aq)+ 2 K3PO4(aq) Mg3(PO4)2(s)+ 6 KCl(aq)
A) MgCl2(aq)+ K3PO4(aq) K3Mg(s)+ PO4Cl2(aq)
B) 3 MgCl2(aq)+ 2 K3PO4(aq) 3 K2Mg(s)+ 2 PO4Cl3(aq)
C) MgCl(aq)+ KPO4(aq) MgPO4(s)+ KCl(aq)
D) MgCl2(aq)+ 2 KPO4(aq) Mg(PO4)2(s)+ 2 KCl(aq)
E) 3 MgCl2(aq)+ 2 K3PO4(aq) Mg3(PO4)2(s)+ 6 KCl(aq)

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
25
All of the following compounds are in water except ____.
A) RuCO3
B) K2CO3
C) (NH4)2CO3
D) Cs2CO3
E) Li2CO3
A) RuCO3
B) K2CO3
C) (NH4)2CO3
D) Cs2CO3
E) Li2CO3

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following would be depicted as the individual ions on the reactant side of a complete ionic reaction?
A) NaOH
B) HCl
C) Cd(NO3)2
D) CdCO3
E) CrCl3
A) NaOH
B) HCl
C) Cd(NO3)2
D) CdCO3
E) CrCl3

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
27
If an aqueous solution of ____ is added to a mixture of Pb2+ and Ba2+,the lead ion will precipitate,but the barium ion will remain in solution.
A) NaOH
B) Na2SO4
C) K3PO4
D) KCO3
E) Ca(CH3CO2)2
A) NaOH
B) Na2SO4
C) K3PO4
D) KCO3
E) Ca(CH3CO2)2

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
28
Which anion will form a precipitate with Li+?
A) Cl-
B) SO42-
C) C2H3O2-
D) S2-
E) none
A) Cl-
B) SO42-
C) C2H3O2-
D) S2-
E) none

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following reactions best describes the dissolution of solid LiOH(s)in water?
A) LiOH(s)+ H2O(l) LiO-(aq)+ H3O+(aq)
B) LiOH(s) LiO-(aq)+ H+(aq)
C) LiOH(s) Li+(aq)+ OH-(aq)
D) LiOH(s) LiO+(aq)+ H-(aq)
E) LiOH(s) LiOH(aq)
A) LiOH(s)+ H2O(l) LiO-(aq)+ H3O+(aq)
B) LiOH(s) LiO-(aq)+ H+(aq)
C) LiOH(s) Li+(aq)+ OH-(aq)
D) LiOH(s) LiO+(aq)+ H-(aq)
E) LiOH(s) LiOH(aq)

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following compounds is in water?
A) Al2S3
B) K2O
C) CoS
D) Hg2I2
E) HgO
A) Al2S3
B) K2O
C) CoS
D) Hg2I2
E) HgO

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
31
What is the net ionic equation for the reaction of aqueous sodium hydroxide and aqueous iron(II)chloride?
A) Na+(aq)+ OH-(aq) NaOH(s)
B) Na+(aq)+ Cl-(aq) NaCl(s)
C) Fe2+(aq)+ 2 OH-(aq) Fe(OH)2(s)
D) Fe2+(aq)+ OH-(aq) FeOH+(s)
E) Fe2+(aq)+ 2 Cl-(aq) FeCl2(s)
A) Na+(aq)+ OH-(aq) NaOH(s)
B) Na+(aq)+ Cl-(aq) NaCl(s)
C) Fe2+(aq)+ 2 OH-(aq) Fe(OH)2(s)
D) Fe2+(aq)+ OH-(aq) FeOH+(s)
E) Fe2+(aq)+ 2 Cl-(aq) FeCl2(s)

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following combinations will produce a precipitate?
1)AgNO3(aq)and HCl(aq)
2)FeCl3(aq)and Na2CO3(aq)
3)NaOH(aq)and K3PO4(aq)
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
1)AgNO3(aq)and HCl(aq)
2)FeCl3(aq)and Na2CO3(aq)
3)NaOH(aq)and K3PO4(aq)
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
33
All of the following compounds are in water except ____.
A) BaCO3
B) PbF2
C) Fe(OH)3
D) Ni(ClO4)2
E) PbCrO4
A) BaCO3
B) PbF2
C) Fe(OH)3
D) Ni(ClO4)2
E) PbCrO4

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
34
Which equation best represents the balanced c equation for the reaction that occurs when aqueous solutions of sodium phosphate and iron(II)nitrate are mixed?
A) 3Fe2+(aq)+ 2PO43-(aq) Fe3(PO4)2(s)
B) 2Na+(aq)+ Fe(NO3)2(aq) 2NaNO3(aq)+ Fe2+(aq)
C) 3Fe2+(aq)+ 2PO43-(aq) Fe3(PO4)2(aq)
D) 2Na3PO4(aq)+ 3Fe2+(aq) Fe3(PO4)2(s)+ (Na+)6(aq)
E) 2Na3PO4(aq)+ 3Fe(NO3)2(aq) Fe3(PO4)2(s)+ 6NaNO3(aq)
A) 3Fe2+(aq)+ 2PO43-(aq) Fe3(PO4)2(s)
B) 2Na+(aq)+ Fe(NO3)2(aq) 2NaNO3(aq)+ Fe2+(aq)
C) 3Fe2+(aq)+ 2PO43-(aq) Fe3(PO4)2(aq)
D) 2Na3PO4(aq)+ 3Fe2+(aq) Fe3(PO4)2(s)+ (Na+)6(aq)
E) 2Na3PO4(aq)+ 3Fe(NO3)2(aq) Fe3(PO4)2(s)+ 6NaNO3(aq)

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
35
Which anion will form a precipitate with Ca2+?
A) Cl-
B) OH-
C) C2H3O2-
D) Br-
E) none
A) Cl-
B) OH-
C) C2H3O2-
D) Br-
E) none

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following ions is most likely to form an compound when combined with sulfate ion?
A) Pb2+
B) F-
C) K+
D) Li+
E) S2-
A) Pb2+
B) F-
C) K+
D) Li+
E) S2-

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
37
A precipitate will form when aqueous Pb(NO3)2 is added to an aqueous solution of ____.
A) Cu(NO3)2
B) NaI
C) NaCH3CO2
D) Pb(ClO4)2
E) KNO3
A) Cu(NO3)2
B) NaI
C) NaCH3CO2
D) Pb(ClO4)2
E) KNO3

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
38
Write a balanced chemical equation for the reaction of aqueous solutions of sodium sulfide and zinc(II)chloride.
A) Na2S(aq)+ ZnCl2(aq) ZnS(s)+ 2 NaCl(aq)
B) Na2S(aq)+ ZnCl2(aq) ZnS(s)+ 2 NaCl(s)
C) Na2S(aq)+ ZnCl2(aq) Na2Zn(s)+ SCl2(aq)
D) Na2S(aq)+ ZnCl2(aq) Na2Zn(aq)+ SCl2(g)
E) No reaction occurs.
A) Na2S(aq)+ ZnCl2(aq) ZnS(s)+ 2 NaCl(aq)
B) Na2S(aq)+ ZnCl2(aq) ZnS(s)+ 2 NaCl(s)
C) Na2S(aq)+ ZnCl2(aq) Na2Zn(s)+ SCl2(aq)
D) Na2S(aq)+ ZnCl2(aq) Na2Zn(aq)+ SCl2(g)
E) No reaction occurs.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
39
If an aqueous solution of ____ is added to a mixture of F- and SO42-,the fluoride ion will precipitate,but the sulfate ion will remain in solution.
A) LiBr
B) HNO3
C) Pb(ClO4)2
D) MgNO3
E) AlCl3
A) LiBr
B) HNO3
C) Pb(ClO4)2
D) MgNO3
E) AlCl3

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
40
A precipitate will form when aqueous nickel(II)chloride is added to an aqueous solution of ____.
A) SrI2
B) Cu(NO3)2
C) KOH
D) Na2SO4
E) NaBr
A) SrI2
B) Cu(NO3)2
C) KOH
D) Na2SO4
E) NaBr

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following is a in aqueous solution?
A) HOCH2CH2OH
B) Sr(OH)2
C) H3PO4
D) NH3
E) HI
A) HOCH2CH2OH
B) Sr(OH)2
C) H3PO4
D) NH3
E) HI

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
42
Hydrocyanic acid,HCN,is a weak acid.Write a net ionic equation for the reaction of aqueous hydrocyanic acid and aqueous sodium hydroxide.
A) HCN(aq)+ NaOH(aq) Na+(aq)+ CN-(aq)+ H2O( )
)
B) HCN(aq)+ H2O(aq) CN-(aq)+ H3O+(aq)
C) H+(aq)+ OH-(aq) H2O( )
)
D) HCN(aq)+ OH-(aq) CN-(aq)+ H2O( )
)
E) H+(aq)+ NaOH(aq) Na+(aq)+ H2O( )
)
A) HCN(aq)+ NaOH(aq) Na+(aq)+ CN-(aq)+ H2O(
 )
)B) HCN(aq)+ H2O(aq) CN-(aq)+ H3O+(aq)
C) H+(aq)+ OH-(aq) H2O(
 )
)D) HCN(aq)+ OH-(aq) CN-(aq)+ H2O(
 )
)E) H+(aq)+ NaOH(aq) Na+(aq)+ H2O(
 )
)
Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
43
What is the balanced equation for carbonate ion (CO32-)acting as a Brønsted base in a reaction with water?
A) CO32-(aq)+ 3 H2O( )
)  CO44-(aq)+ 2 H3O+(aq)
CO44-(aq)+ 2 H3O+(aq)
B) CO32-(aq)+ H2O( )
)  HCO3-(aq)+ OH-(aq)
HCO3-(aq)+ OH-(aq)
C) CO32-(aq)+ H2O(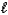 )
)  CO2(g)+ 2 OH-(aq)
CO2(g)+ 2 OH-(aq)
D) CO32-(aq)+ 2 H2O(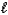 )
)  HCO3-(aq)+ H3O+(aq)
HCO3-(aq)+ H3O+(aq)
E) CO32-(aq)+ H2O( )
)  H2CO42-(aq)
H2CO42-(aq)
A) CO32-(aq)+ 3 H2O(
 )
)  CO44-(aq)+ 2 H3O+(aq)
CO44-(aq)+ 2 H3O+(aq)B) CO32-(aq)+ H2O(
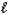 )
)  HCO3-(aq)+ OH-(aq)
HCO3-(aq)+ OH-(aq)C) CO32-(aq)+ H2O(
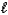 )
)  CO2(g)+ 2 OH-(aq)
CO2(g)+ 2 OH-(aq)D) CO32-(aq)+ 2 H2O(
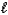 )
)  HCO3-(aq)+ H3O+(aq)
HCO3-(aq)+ H3O+(aq)E) CO32-(aq)+ H2O(
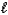 )
)  H2CO42-(aq)
H2CO42-(aq)
Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following is a weak electrolyte in aqueous solution?
A) Mg(OH)2
B) NH3
C) NaOH
D) KOH
E) Ba(OH)2
A) Mg(OH)2
B) NH3
C) NaOH
D) KOH
E) Ba(OH)2

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
45
What are the spectator ions in the reaction between aqueous hydrochloric acid and aqueous potassium hydroxide?
A) K+ only
B) H+ and OH-
C) K+ and Cl-
D) Cl- only
E) H+,Cl-,K+,and OH-
A) K+ only
B) H+ and OH-
C) K+ and Cl-
D) Cl- only
E) H+,Cl-,K+,and OH-

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following compounds will produce a basic solution when dissolved in water?
A) CaO
B) NaHSO4
C) CO2
D) SO2
E) KCl
A) CaO
B) NaHSO4
C) CO2
D) SO2
E) KCl

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following is a in aqueous solution?
A) LiCl
B) LiOH
C) NH3
D) HBr
E) Ca(OH)2
A) LiCl
B) LiOH
C) NH3
D) HBr
E) Ca(OH)2

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following compounds is a in aqueous solution?
A) HCl
B) H3PO4
C) HNO3
D) HClO4
E) H2SO4
A) HCl
B) H3PO4
C) HNO3
D) HClO4
E) H2SO4

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following would be depicted as the individual ions on the reactant side of a complete ionic reaction?
A) RbOH
B) HBr
C) ZnBr2
D) CH3COOH
E) MnCl3
A) RbOH
B) HBr
C) ZnBr2
D) CH3COOH
E) MnCl3

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
50
What is the net ionic equation for the neutralization of hydroiodic acid with aqueous potassium hydroxide?
A) H+(aq)+ OH-(aq) H2O( )
)
B) H+(aq)+ I-(aq)+ K+(aq)+ OH-(aq) KI(aq)+ H2O( )
)
C) H+(aq)+ I-(aq)+ K+(aq)+ OH-(aq) HI(aq)+ KOH(aq)
D) HI(aq)+ K+(aq)+ OH-(aq) KI(aq)+ H2O( )
)
E) HI(aq)+ KOH(aq) KI(aq)+ H2O( )
)
A) H+(aq)+ OH-(aq) H2O(
 )
)B) H+(aq)+ I-(aq)+ K+(aq)+ OH-(aq) KI(aq)+ H2O(
 )
)C) H+(aq)+ I-(aq)+ K+(aq)+ OH-(aq) HI(aq)+ KOH(aq)
D) HI(aq)+ K+(aq)+ OH-(aq) KI(aq)+ H2O(
 )
)E) HI(aq)+ KOH(aq) KI(aq)+ H2O(
 )
)
Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following is a in aqueous solution?
A) HOCH2CH2OH
B) Mg(OH)2
C) H3PO4
D) NH3
E) HNO3
A) HOCH2CH2OH
B) Mg(OH)2
C) H3PO4
D) NH3
E) HNO3

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
52
When solutions of barium chloride and lithium sulfate are mixed,the in the resulting precipitation reaction are
A) only SO42-.
B) both Li+ and Cl-.
C) only Cl-.
D) only Li+.
E) only Ba2+.
A) only SO42-.
B) both Li+ and Cl-.
C) only Cl-.
D) only Li+.
E) only Ba2+.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
53
Which equation best represents the that occurs when an aqueous solution of barium bromide is mixed with an aqueous solution of sodium sulfate?
A) 2H+(aq)+ 2Br-(aq) 2HBr(g)
B) Ba2+(aq)+ SO42-(aq) BaSO4(s)
C) Ba2+(aq)+ 2Br-(aq)+ 2Na+(aq)+ SO42-(aq) BaSO4(s)+ 2NaBr(aq)
D) BaBr2(aq)+ Na2SO4(aq) BaSO4(s)+ 2NaBr(aq)
E) No net reaction occurs.
A) 2H+(aq)+ 2Br-(aq) 2HBr(g)
B) Ba2+(aq)+ SO42-(aq) BaSO4(s)
C) Ba2+(aq)+ 2Br-(aq)+ 2Na+(aq)+ SO42-(aq) BaSO4(s)+ 2NaBr(aq)
D) BaBr2(aq)+ Na2SO4(aq) BaSO4(s)+ 2NaBr(aq)
E) No net reaction occurs.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
54
Write a balanced equation for the reaction that occurs when hydrogen sulfate ion behaves as a Brønsted-Lowry acid in water.
A) HSO4-(aq)+ H2O( )
)  H2SO4(aq)+ H3O+(aq)
H2SO4(aq)+ H3O+(aq)
B) SO42-(aq)+ H2O( )
)  HSO4-(aq)+ OH-(aq)
HSO4-(aq)+ OH-(aq)
C) HSO4-(aq)+ H2O( )
)  SO42-(aq)+ H3O+(aq)
SO42-(aq)+ H3O+(aq)
D) HSO4-(aq)+ H2O( )
)  H2SO4(aq)+ OH-(aq)
H2SO4(aq)+ OH-(aq)
E) H2SO4(aq)+ H2O( )
)  HSO4-(aq)+ H3O+(aq)
HSO4-(aq)+ H3O+(aq)
A) HSO4-(aq)+ H2O(
 )
)  H2SO4(aq)+ H3O+(aq)
H2SO4(aq)+ H3O+(aq)B) SO42-(aq)+ H2O(
 )
)  HSO4-(aq)+ OH-(aq)
HSO4-(aq)+ OH-(aq)C) HSO4-(aq)+ H2O(
 )
)  SO42-(aq)+ H3O+(aq)
SO42-(aq)+ H3O+(aq)D) HSO4-(aq)+ H2O(
 )
)  H2SO4(aq)+ OH-(aq)
H2SO4(aq)+ OH-(aq)E) H2SO4(aq)+ H2O(
 )
)  HSO4-(aq)+ H3O+(aq)
HSO4-(aq)+ H3O+(aq)
Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
55
What are the spectator ions in the reaction between aqueous nitric acid and ammonia?
A) H+ only
B) NO3- only
C) H+ and NH4+
D) NO3- and NH4+
E) H+,NO3-,and NH4+
A) H+ only
B) NO3- only
C) H+ and NH4+
D) NO3- and NH4+
E) H+,NO3-,and NH4+

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
56
Metal oxides react with water to produce ____.
A) bases
B) hydrogen gas
C) oxygen gas
D) acids
E) hydronium ions
A) bases
B) hydrogen gas
C) oxygen gas
D) acids
E) hydronium ions

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
57
What base results when Li2O reacts with water?
A) H3O+(aq)
B) LiH(aq)
C) O2-(aq)
D) LiOH(aq)
E) LiO-(aq)
A) H3O+(aq)
B) LiH(aq)
C) O2-(aq)
D) LiOH(aq)
E) LiO-(aq)

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
58
In the reaction of acetic acid with aqueous cesium hydroxide,what is the spectator ion?
A) OH-(aq)
B) There is no spectator ion.
C) C2H3O2-(aq)
D) Cs+(aq)
E) H+(aq)
A) OH-(aq)
B) There is no spectator ion.
C) C2H3O2-(aq)
D) Cs+(aq)
E) H+(aq)

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
59
Nitric acid is the product of the reaction of ____ and H2O.
A) SO3
B) NO2
C) N2
D) NH3
E) N3-
A) SO3
B) NO2
C) N2
D) NH3
E) N3-

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
60
The net ionic equation for the reaction between aqueous ammonia and hydrochloric acid is
A) HCl(aq)+ NH3(aq) NH4Cl(aq).
B) H+(aq)+ OH-(aq) H2O( ).
).
C) HCl(aq)+ OH-(aq) Cl-(aq)+ H2O( ).
).
D) H+(aq)+ NH3(aq) NH4+(aq).
E) H+(aq)+ Cl-(aq)+ NH3(aq) NH4+(aq)+ Cl-(aq).
A) HCl(aq)+ NH3(aq) NH4Cl(aq).
B) H+(aq)+ OH-(aq) H2O(
 ).
).C) HCl(aq)+ OH-(aq) Cl-(aq)+ H2O(
 ).
).D) H+(aq)+ NH3(aq) NH4+(aq).
E) H+(aq)+ Cl-(aq)+ NH3(aq) NH4+(aq)+ Cl-(aq).

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
61
Which species in the reaction below undergoes reduction?
H2O(g)+ CO(g) H2(g)+ CO2(g)
A) H2O
B) CO
C) H2
D) CO2
E) None
H2O(g)+ CO(g) H2(g)+ CO2(g)
A) H2O
B) CO
C) H2
D) CO2
E) None

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
62
A(n)________ agent gains electrons in an oxidation-reduction reaction.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following chemical equations show oxidation-reduction reactions?
1)Mg(s)+ I2(aq) MgI2(s)
2)Pb(ClO4)2(aq)+ 2 KI(aq) PbI2(s)+ 2 KClO4(aq)
3)Fe2O3(s)+ 3 CO(g) 2 Fe(s)+ 3 CO2(g)
A) 1 only
B) 2 only
C) 1 and 2
D) 1 and 3
E) 2 and 3
1)Mg(s)+ I2(aq) MgI2(s)
2)Pb(ClO4)2(aq)+ 2 KI(aq) PbI2(s)+ 2 KClO4(aq)
3)Fe2O3(s)+ 3 CO(g) 2 Fe(s)+ 3 CO2(g)
A) 1 only
B) 2 only
C) 1 and 2
D) 1 and 3
E) 2 and 3

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
64
Write a balanced chemical equation for the reaction of aqueous solutions of ammonium sulfate and sodium hydroxide.
A) (NH4)2SO4(aq)+ 2 NaOH(aq) 2 NH4OH(aq)+ SO3(g)+ Na2O(aq)
B) (NH3)2SO4(aq)+ NaOH(aq) 2 NH3(g)+ NaOHSO4(aq)
C) (NH3)2SO4(aq)+ 2 NaOH(aq) 2 NH3(g)+ Na2SO4(aq)+ 2 OH-(aq)
D) (NH4)2SO4(aq)+ 2 NaOH(aq) 2 NH4+(g)+ Na2SO4(aq)+ 2 OH-(aq)
E) (NH4)2SO4(aq)+ 2 NaOH(aq) 2 NH3(g)+ 2 H2O( )+ Na2SO4(aq)
)+ Na2SO4(aq)
A) (NH4)2SO4(aq)+ 2 NaOH(aq) 2 NH4OH(aq)+ SO3(g)+ Na2O(aq)
B) (NH3)2SO4(aq)+ NaOH(aq) 2 NH3(g)+ NaOHSO4(aq)
C) (NH3)2SO4(aq)+ 2 NaOH(aq) 2 NH3(g)+ Na2SO4(aq)+ 2 OH-(aq)
D) (NH4)2SO4(aq)+ 2 NaOH(aq) 2 NH4+(g)+ Na2SO4(aq)+ 2 OH-(aq)
E) (NH4)2SO4(aq)+ 2 NaOH(aq) 2 NH3(g)+ 2 H2O(
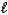 )+ Na2SO4(aq)
)+ Na2SO4(aq)
Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
65
Which species is reduced in the reaction below?
I-(aq)+ ClO-(aq) IO-(aq)+ Cl-(aq)
A) I-
B) H2O
C) Cl-
D) IO-
E) ClO-
I-(aq)+ ClO-(aq) IO-(aq)+ Cl-(aq)
A) I-
B) H2O
C) Cl-
D) IO-
E) ClO-

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following are classified as precipitation reactions?
1)AgNO3(aq)+ HCl(aq) AgCl(s)+ HNO3(aq)
2)2Mg(s)+ O2(g) 2MgO(s)
3)Zn(s)+ 2Ag+(aq) 2Ag(s)+ Zn2+(aq)
A) 1 only
B) 2 only
C) 3 only
D) 2 and 3
E) 1,2,and 3
1)AgNO3(aq)+ HCl(aq) AgCl(s)+ HNO3(aq)
2)2Mg(s)+ O2(g) 2MgO(s)
3)Zn(s)+ 2Ag+(aq) 2Ag(s)+ Zn2+(aq)
A) 1 only
B) 2 only
C) 3 only
D) 2 and 3
E) 1,2,and 3

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
67
In the following reaction,which species is oxidized?
3Rb2S(s)+ 8H+(aq)+ 2NO3-(aq) 6Rb+(aq)+ 3S(s)+ 2NO(g)+ 4H2O
A) NO3-
B) Rb2S
C) Rb+
D) NO
E) H+
3Rb2S(s)+ 8H+(aq)+ 2NO3-(aq) 6Rb+(aq)+ 3S(s)+ 2NO(g)+ 4H2O
A) NO3-
B) Rb2S
C) Rb+
D) NO
E) H+

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
68
The for the reaction of magnesium sulfite with nitric acid is
A) MgSO3(s)+ 2H+(aq) Mg2+(aq)+ SO2(g)+ H2O(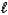 ).
).
B) Mg2+(aq)+ CO32-(aq)+ 2H+(aq)+ 2NO3-(aq) Mg(NO3)2(aq)+ SO2(g)+ H2O(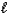 ).
).
C) MgSO3(s)+ 2HNO2(aq) Mg2+(aq)+ 2NO2-(aq)+ SO2(g)+ H2O( ).
).
D) Mg(HSO3)2(s)+ 2HNO3(aq) Mg2+(aq)+ 2NO3-(aq)+ SO2(g)+ 2H2O( ).
).
E) MgSO3(s)+ 2HNO3(aq) Mg2+(aq)+ 2NO3-(aq)+ SO2(g)+ H2O( ).
).
A) MgSO3(s)+ 2H+(aq) Mg2+(aq)+ SO2(g)+ H2O(
 ).
).B) Mg2+(aq)+ CO32-(aq)+ 2H+(aq)+ 2NO3-(aq) Mg(NO3)2(aq)+ SO2(g)+ H2O(
 ).
).C) MgSO3(s)+ 2HNO2(aq) Mg2+(aq)+ 2NO2-(aq)+ SO2(g)+ H2O(
 ).
).D) Mg(HSO3)2(s)+ 2HNO3(aq) Mg2+(aq)+ 2NO3-(aq)+ SO2(g)+ 2H2O(
 ).
).E) MgSO3(s)+ 2HNO3(aq) Mg2+(aq)+ 2NO3-(aq)+ SO2(g)+ H2O(
 ).
).
Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following is/are classified as an acid-base reaction?
1.AgNO3(aq)+ HCl(aq) AgCl(s)+ HNO3(aq)
2.2Mg(s)+ O2(g) 2MgO(s)
3.Zn(s)+ 2Ag+(aq) 2Ag(s)+ Zn2+(aq)
A)1 only
B)2 only
C)3 only
D)1,2,and 3
E)none
1.AgNO3(aq)+ HCl(aq) AgCl(s)+ HNO3(aq)
2.2Mg(s)+ O2(g) 2MgO(s)
3.Zn(s)+ 2Ag+(aq) 2Ag(s)+ Zn2+(aq)
A)1 only
B)2 only
C)3 only
D)1,2,and 3
E)none

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
70
The net ionic equation for the reaction of barium chloride and sodium sulfate is shown below.Ba2+(aq)+ SO42-(aq) BaSO4(s)
Chloride and sodium ions are referred to as ________ ions because they are not involved in the reaction.
Chloride and sodium ions are referred to as ________ ions because they are not involved in the reaction.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
71
The oxidation number of nitrogen is highest in which of the following?
A) NH3
B) N2
C) HNO2
D) NaNO3
E) NO2-
A) NH3
B) N2
C) HNO2
D) NaNO3
E) NO2-

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
72
What is the oxidation number of each atom in sodium hydrogen carbonate,NaHCO3?
A) Na = +1, H = -1, C = +6,O = -2
B) Na = +1, H = +1, C = +4,O = -2
C) Na = +1, H = -1, C = +2,O = -2
D) Na = -1, H = +1, C = 0,O = -2
E) Na = 0, H = 0, C = 0,O = 0
A) Na = +1, H = -1, C = +6,O = -2
B) Na = +1, H = +1, C = +4,O = -2
C) Na = +1, H = -1, C = +2,O = -2
D) Na = -1, H = +1, C = 0,O = -2
E) Na = 0, H = 0, C = 0,O = 0

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
73
________ acid is produced in a larger quantity than any other chemical in the United States.This chemical is used in the production of fertilizers,pigments,alcohol,paper and detergents.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
74
Which molecule in the reaction below is the oxidizing agent?
2 C2H6(g)+ 7 O2(g) 4 CO2(g)+ 6 H2O(g)
A) C2H6
B) O2
C) H2O
D) CO2
E) None
2 C2H6(g)+ 7 O2(g) 4 CO2(g)+ 6 H2O(g)
A) C2H6
B) O2
C) H2O
D) CO2
E) None

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
75
Write a balanced net ionic equation for the reaction of aqueous solutions of baking soda (NaHCO3)and acetic acid.
A) HCO3-(aq)+ CH3CO2H(aq) CH3CO2-(aq)+ H2O( )+ CO2(g)
)+ CO2(g)
B) 2 NaHCO3(aq)+ CH3CO2H(aq) 2 Na2CO3(aq)+ CH4(aq)+ 2H2O( )+ CO2(g)
)+ CO2(g)
C) NaHCO3(aq)+ H+(aq) H2CO3(s)+ Na+(aq)
D) HCO3-(aq)+ H+(aq) H2O( )+ CO2(g)
)+ CO2(g)
E) HCO3-(aq)+ H+(aq) H2CO3(aq)
A) HCO3-(aq)+ CH3CO2H(aq) CH3CO2-(aq)+ H2O(
 )+ CO2(g)
)+ CO2(g)B) 2 NaHCO3(aq)+ CH3CO2H(aq) 2 Na2CO3(aq)+ CH4(aq)+ 2H2O(
 )+ CO2(g)
)+ CO2(g)C) NaHCO3(aq)+ H+(aq) H2CO3(s)+ Na+(aq)
D) HCO3-(aq)+ H+(aq) H2O(
 )+ CO2(g)
)+ CO2(g)E) HCO3-(aq)+ H+(aq) H2CO3(aq)

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following chemical equations is an acid-base reaction?
A) Ba(OH)2(aq)+ K2SO4(aq) BaSO4(s)+ 2 KOH(aq)
B) 3 NaOH(aq)+ AlCl3(aq) Al(OH)3(s)+ 3 NaCl(aq)
C) 2 H+(aq)+ Zn(s) H2(g)+ Zn2+(aq)
D) 2 HCl(aq)+ Pb(NO3)2(aq) PbCl2(s)+ 2 HNO3(aq)
E) H3PO4(aq)+ NH3(aq) NH4+(aq)+ H2PO4-(aq)
A) Ba(OH)2(aq)+ K2SO4(aq) BaSO4(s)+ 2 KOH(aq)
B) 3 NaOH(aq)+ AlCl3(aq) Al(OH)3(s)+ 3 NaCl(aq)
C) 2 H+(aq)+ Zn(s) H2(g)+ Zn2+(aq)
D) 2 HCl(aq)+ Pb(NO3)2(aq) PbCl2(s)+ 2 HNO3(aq)
E) H3PO4(aq)+ NH3(aq) NH4+(aq)+ H2PO4-(aq)

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
77
All of the following are oxidation-reduction reactions EXCEPT
A) CaCO3(s) CaO(s)+ CO2(g)
B) 2 Na(s)+ Br2(g) 2 NaBr(g)
C) Fe(s)+ 2 HCl(aq) FeCl2(aq)+ H2(g)
D) 2 C(s)+ O2(g) 2 CO(g)
E) 2 H2O( ) 2 H2(g)+ O2(g)
) 2 H2(g)+ O2(g)
A) CaCO3(s) CaO(s)+ CO2(g)
B) 2 Na(s)+ Br2(g) 2 NaBr(g)
C) Fe(s)+ 2 HCl(aq) FeCl2(aq)+ H2(g)
D) 2 C(s)+ O2(g) 2 CO(g)
E) 2 H2O(
 ) 2 H2(g)+ O2(g)
) 2 H2(g)+ O2(g)
Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
78
What is the oxidation number of each O in NH4(H2PO4)?
A) -3
B) +1
C) -2
D) +5
E) 0
A) -3
B) +1
C) -2
D) +5
E) 0

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
79
What is the oxidation number of each O in BaFeO4?
A) +6
B) +2
C) -2
D) +3
E) 0
A) +6
B) +2
C) -2
D) +3
E) 0

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following elements generally acts as an oxidizing agent?
A) Br2
B) H2
C) Fe
D) C
E) Li
A) Br2
B) H2
C) Fe
D) C
E) Li

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck



