Deck 13: Nuclear Changes and Nuclear Power
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/73
Play
Full screen (f)
Deck 13: Nuclear Changes and Nuclear Power
1
The product of the beta ( 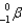 ) decay of thorium-234 is
) decay of thorium-234 is
A) uranium-238.
B) protactinium-234.
C) uranium-234.
D) uranium-235.
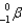 ) decay of thorium-234 is
) decay of thorium-234 isA) uranium-238.
B) protactinium-234.
C) uranium-234.
D) uranium-235.
protactinium-234.
2
What is the nucleus produced when beryllium-9, ( 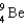 ), fuses with helium-4?
), fuses with helium-4?
A) oxygen-17
B) carbon-13
C) carbon-14
D) carbon-12
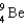 ), fuses with helium-4?
), fuses with helium-4?A) oxygen-17
B) carbon-13
C) carbon-14
D) carbon-12
carbon-13
3
Which type of radiation is the most penetrating?
A) gamma
B) alpha
C) beta
D) all have about the same penetrating power
A) gamma
B) alpha
C) beta
D) all have about the same penetrating power
gamma
4
Radon in the air arises from
A) sun's ultraviolet radiation that enters earth's atmosphere.
B) radioactive decay of uranium in the soil.
C) increased use of microwave devices by humans.
D) radiation produced by worldwide use of electrical power lines.
A) sun's ultraviolet radiation that enters earth's atmosphere.
B) radioactive decay of uranium in the soil.
C) increased use of microwave devices by humans.
D) radiation produced by worldwide use of electrical power lines.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
5
Cobalt-60
A) is used to treat cancerous tumors.
B) decays to both beta particles and gamma rays.
C) is a relatively stable isotope.
D) All of the above characterize cobalt-60.
A) is used to treat cancerous tumors.
B) decays to both beta particles and gamma rays.
C) is a relatively stable isotope.
D) All of the above characterize cobalt-60.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
6
Who first discovered radioactivity?
A) Ernest Rutherford
B) Max Planck
C) Marie Curie
D) Henri Becquerel
A) Ernest Rutherford
B) Max Planck
C) Marie Curie
D) Henri Becquerel

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
7
What type of particle is emitted when a U-235 decays to Np-235?
A) alpha particle
B) beta particle
C) neutron
D) helium nuclei
A) alpha particle
B) beta particle
C) neutron
D) helium nuclei

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
8
Stable nuclei (that is, nonradioactive nuclei) have mass numbers that are
A) equal to the atomic number.
B) twice as large as the atomic number or even larger.
C) ten times the atomic number.
D) less than twice as large as the atomic number.
A) equal to the atomic number.
B) twice as large as the atomic number or even larger.
C) ten times the atomic number.
D) less than twice as large as the atomic number.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
9
Which is larger in terms of radioactive disintegrations per second?
A) one millicurie
B) one microcurie
C) one curie
D) one picocurie
A) one millicurie
B) one microcurie
C) one curie
D) one picocurie

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
10
In the symbol 238U, the 238 is
A) the mass number.
B) the atomic number.
C) the number of neutrons in the atom.
D) the number of protons in the atom.
A) the mass number.
B) the atomic number.
C) the number of neutrons in the atom.
D) the number of protons in the atom.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
11
To make an alpha particle fuse with the nucleus of another atom, you need to
A) aim carefully.
B) give the alpha particle a negative charge.
C) give the alpha particle a large amount of energy.
D) slow the particles down by cooling.
A) aim carefully.
B) give the alpha particle a negative charge.
C) give the alpha particle a large amount of energy.
D) slow the particles down by cooling.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
12
Who was first to produce and describe an artificial nuclear reaction with the bombardment of nitrogen with alpha particles?
A) Geiger
B) Wilson
C) Rutherford
D) Chadwick
A) Geiger
B) Wilson
C) Rutherford
D) Chadwick

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
13
The product of the alpha ( 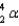 ) decay of uranium-238 is
) decay of uranium-238 is
A) Th-234.
B) Th-230.
C) U-234.
D) U-242.
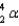 ) decay of uranium-238 is
) decay of uranium-238 isA) Th-234.
B) Th-230.
C) U-234.
D) U-242.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
14
The nuclear reaction that changes one element into another element is called a
A) chemical reaction.
B) half-life.
C) transmutation.
D) decay series.
A) chemical reaction.
B) half-life.
C) transmutation.
D) decay series.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
15
If the half-life of an element is 5 days and 100 milligrams of that element is initially available, how many milligrams of the element are present after 25 days?
A) 50 mg
B) 100/25 mg
C) 100/32 mg
D) 100/64 mg
A) 50 mg
B) 100/25 mg
C) 100/32 mg
D) 100/64 mg

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
16
What is the ultimate radioactive decay product of the uranium-238 series?
A) radon gas
B) lead
C) uranium-235
D) alpha particles
A) radon gas
B) lead
C) uranium-235
D) alpha particles

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
17
How many atoms of zinc-69 remain after a sample of 500,000 zinc-69 atoms decays for a period of 165 minutes? The half-life time for zinc-69 is 55 minutes.
A) 500,000 atoms
B) 62,500 atoms
C) 250,000 atoms
D) 125,000 atoms
A) 500,000 atoms
B) 62,500 atoms
C) 250,000 atoms
D) 125,000 atoms

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
18
Which is generally not associated with nuclear reactions?
A) outer electrons
B) atomic number
C) atomic mass
D) the nucleus
A) outer electrons
B) atomic number
C) atomic mass
D) the nucleus

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
19
What is the "stable" nucleus produced at the end of the decay steps in the uranium decay series?
A) thorium
B) uranium
C) radon
D) lead
A) thorium
B) uranium
C) radon
D) lead

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
20
The first transuranium element to be discovered was
A) Np.
B) Pu.
C) Hf.
D) Am.
A) Np.
B) Pu.
C) Hf.
D) Am.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
21
A major, unsolved problem with nuclear energy is
A) cost.
B) engineering problems with design reactors.
C) acquiring land on which to build nuclear reactors.
D) what to do with nuclear wastes with long half-lives.
A) cost.
B) engineering problems with design reactors.
C) acquiring land on which to build nuclear reactors.
D) what to do with nuclear wastes with long half-lives.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
22
What produces the tremendous energy of a nuclear fission reaction?
A) some mass being changed into energy
B) the splitting of the nucleus
C) the extra neutrons produced
D) disintegration of the containment building
A) some mass being changed into energy
B) the splitting of the nucleus
C) the extra neutrons produced
D) disintegration of the containment building

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
23
Which is true about fusion?
A) Very high temperatures are required.
B) Fusion occurs in a plasma of charged particles.
C) Fusion produces fewer radioactive byproducts.
D) All statements are true.
A) Very high temperatures are required.
B) Fusion occurs in a plasma of charged particles.
C) Fusion produces fewer radioactive byproducts.
D) All statements are true.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
24
In 1934, when the Curies bombarded Al-27 with an alpha particle ( 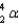 ), the element produced was
), the element produced was
A) silicon-29 and a neutron.
B) phosphorus-30 and a neutron.
C) silicon-29 and a positron.
D) phosphorus-30 and a positron.
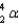 ), the element produced was
), the element produced wasA) silicon-29 and a neutron.
B) phosphorus-30 and a neutron.
C) silicon-29 and a positron.
D) phosphorus-30 and a positron.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
25
The missing nucleus in the equation shown below is 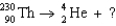
A)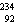 U.
U.
B)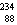 Ra.
Ra.
C)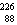 Ra.
Ra.
D)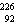 U.
U.
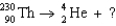
A)
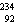 U.
U.B)
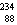 Ra.
Ra.C)
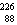 Ra.
Ra.D)
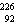 U.
U.
Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
26
When the nucleus of an atom of uranium-238 emits an alpha particle, the mass number will
A) decrease by 4 units.
B) decrease by 2 units.
C) increase by 4 units.
D) increase by 2 units.
A) decrease by 4 units.
B) decrease by 2 units.
C) increase by 4 units.
D) increase by 2 units.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
27
The binding energy of a nucleus is
A) the energy emitted when separate neutrons, protons, and electrons combine to form an atom.
B) the energy required to dissociate an atom into separate neutrons, protons, and neutrons.
C) equivalent to a quantity of mass expressed by Einstein's equation, E = mc2.
D) All of the preceding answers are correct.
A) the energy emitted when separate neutrons, protons, and electrons combine to form an atom.
B) the energy required to dissociate an atom into separate neutrons, protons, and neutrons.
C) equivalent to a quantity of mass expressed by Einstein's equation, E = mc2.
D) All of the preceding answers are correct.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
28
Tritium, H-3, and hydrogen, H-1, are
A) fission reactants.
B) fission products.
C) fusion reactants.
D) fusion products.
A) fission reactants.
B) fission products.
C) fusion reactants.
D) fusion products.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
29
Which statement is not true about fission?
A) combination of atomic nuclei
B) process requires initiation by a neutron
C) a chain reaction
D) products have smaller nuclei than reactants
A) combination of atomic nuclei
B) process requires initiation by a neutron
C) a chain reaction
D) products have smaller nuclei than reactants

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
30
The Food and Drug Administration, FDA, has approved the use of food irradiation to prevent the growth of bacteria, molds, and yeasts in which of the following foods.
A) uncooked beef
B) uncooked pork
C) uncooked poultry
D) all of the above
A) uncooked beef
B) uncooked pork
C) uncooked poultry
D) all of the above

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
31
When a radioactive sample decays for 2 half-lives the amount remaining will be ____ of the original.
A) 1/4
B) 1/2
C) 1/8
D) unpredictable
A) 1/4
B) 1/2
C) 1/8
D) unpredictable

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
32
In terms of nuclear stability, which of the following atoms is the most stable?
A) Fe
B) H
C) U
D) element 109
A) Fe
B) H
C) U
D) element 109

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
33
The fusion of nuclei requires
A) a critical mass.
B) plasma at extremely high temperatures.
C) heavy nuclei.
D) slow moving (thermal) neutrons.
A) a critical mass.
B) plasma at extremely high temperatures.
C) heavy nuclei.
D) slow moving (thermal) neutrons.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
34
The splitting of a nucleus by a slow moving neutron is called
A) fission.
B) fusion.
C) fragmentation.
D) annihilation.
A) fission.
B) fusion.
C) fragmentation.
D) annihilation.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
35
Which component of a nuclear reactor slows the speed of the neutron?
A) shield
B) heat-transfer fluid
C) moderator
D) uranium fuel
A) shield
B) heat-transfer fluid
C) moderator
D) uranium fuel

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
36
Gamma radiation from cobalt-60 or cesium-137 is used in
A) agricultural research.
B) dating charcoal paintings.
C) food irradiation.
D) medical imaging.
A) agricultural research.
B) dating charcoal paintings.
C) food irradiation.
D) medical imaging.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
37
Which is not true about plutonium-239?
A) an unavoidable by-product in nuclear energy production
B) short half-life
C) fissionable
D) used in nuclear war heads
A) an unavoidable by-product in nuclear energy production
B) short half-life
C) fissionable
D) used in nuclear war heads

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
38
The quantity, 37,000 disintegrations per second (dps), is
A) 1 curie.
B) 1 microcurie.
C) half-life of carbon-14.
D) 1 bequerel.
A) 1 curie.
B) 1 microcurie.
C) half-life of carbon-14.
D) 1 bequerel.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
39
Which atom can undergo fission by thermal neutrons?
A) U-235
B) U-238
C) I-131
D) H-3
A) U-235
B) U-238
C) I-131
D) H-3

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following countries generates the least amount of electricity by nuclear fission?
A) France
B) Sweden
C) United States
D) Belgium
A) France
B) Sweden
C) United States
D) Belgium

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
41
The missing nucleus in the equation shown below is 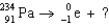
A)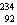 U.
U.
B)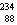 Ra.
Ra.
C)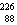 Ra.
Ra.
D)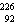 U.
U.
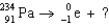
A)
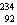 U.
U.B)
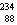 Ra.
Ra.C)
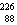 Ra.
Ra.D)
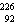 U.
U.
Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following is not a current application of radioactivity?
A) food irradiation
B) radiocarbon dating
C) medical imaging
D) electrical energy from fusion reactions
A) food irradiation
B) radiocarbon dating
C) medical imaging
D) electrical energy from fusion reactions

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following characterizes a gamma ray?
A) is more penetrating than a alpha ray
B) is attracted to the positively charged plate in an electric field
C) is composed of electrons
D) is not deflected by an electric field
E) Two of the above are correct.
A) is more penetrating than a alpha ray
B) is attracted to the positively charged plate in an electric field
C) is composed of electrons
D) is not deflected by an electric field
E) Two of the above are correct.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
44
Consider the following atomic level depiction of a reaction. 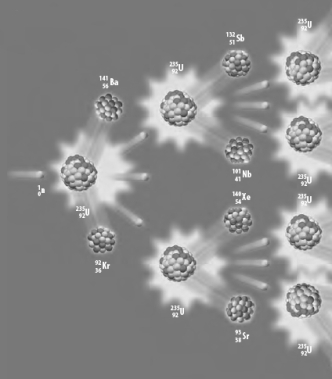
This reaction
A) is a fusion reaction.
B) would be an example of a chain reaction.
C) is initiated by decay.
D) utilizes the most abundant isotope of uranium

This reaction
A) is a fusion reaction.
B) would be an example of a chain reaction.
C) is initiated by decay.
D) utilizes the most abundant isotope of uranium

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
45
The product of the gamma decay of lead-210 is
A) bismuth-210.
B) lead-210.
C) bismuth 209.
D) lead-209.
A) bismuth-210.
B) lead-210.
C) bismuth 209.
D) lead-209.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
46
What type of particle is emitted when a Po-210 decays to Pb-206?
A) alpha particle
B) beta particle
C) neutron
D) helium nuclei
E) either a or d
A) alpha particle
B) beta particle
C) neutron
D) helium nuclei
E) either a or d

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following characterizes an alpha particle?
A) is more penetrating than a gamma ray
B) is attracted to the negitively charge plate in an electric field
C) carries a negative charge
D) is composed of electrons
E) Two of the above are corrected.
A) is more penetrating than a gamma ray
B) is attracted to the negitively charge plate in an electric field
C) carries a negative charge
D) is composed of electrons
E) Two of the above are corrected.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
48
Rank the following types of radiation from least penetrating to most penetrating:
A) " < < "
B) " < < "
C) " < < "
D) " < < "
E) " < < "
A) " < < "
B) " < < "
C) " < < "
D) " < < "
E) " < < "

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
49
Identify the atoms that could be used in nuclear fission and fusion, respectively?
A) C-14 and H-3
B) Rn-222 and H-3
C) Rn-222 and U-238
D) Fe-56 and H-1
A) C-14 and H-3
B) Rn-222 and H-3
C) Rn-222 and U-238
D) Fe-56 and H-1

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
50
When the lead-210 undergoes beta decay, the symbol of the other product of the reaction is:
A)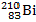
B)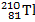
C)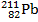
D)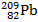
E)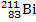
A)
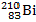
B)
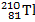
C)
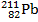
D)
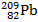
E)
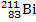

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
51
Is it possible to determine the age of an artifact that is estimated to be 100,000 years old by radiocarbon dating?
A) Yes, because the half-life of carbon is 5730 years
B) No, because the half-life of carbon is only 12 years
C) Yes, because the amount of carbon remaining is very high
D) No, because the amount of carbon remaining is very low
A) Yes, because the half-life of carbon is 5730 years
B) No, because the half-life of carbon is only 12 years
C) Yes, because the amount of carbon remaining is very high
D) No, because the amount of carbon remaining is very low

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
52
Chernobyl is the site of a 1986 nuclear reactor accident that
A) killed 4300 workers who cleaned up the site.
B) occurred near Kiev, Ukraine.
C) occurred when the graphite (carbon) core caught fire.
D) all of these
A) killed 4300 workers who cleaned up the site.
B) occurred near Kiev, Ukraine.
C) occurred when the graphite (carbon) core caught fire.
D) all of these

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
53
Identify the high-level radioactive waste?
A) weapons industry waste
B) laboratory clothing waste
C) medical waste
D) smoke detector waste
A) weapons industry waste
B) laboratory clothing waste
C) medical waste
D) smoke detector waste

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
54
Short half-lives are the results of high rates of decay, and long half-fives are the results of low rates of decay.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
55
Which type of radiation can penetrate a thin piece of lead?
A) gamma
B) alpha
C) beta
D) all the above
A) gamma
B) alpha
C) beta
D) all the above

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
56
If the half-life of an element is 12 years. How much of a 5.00 g sample remains after 36 years?
A) 2.50
B) 1.25
C) 0.625
D) 0.313
A) 2.50
B) 1.25
C) 0.625
D) 0.313

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following characterizes a beta particle?
A) is more penetrating than a gamma ray
B) is attracted to the positively charged plate in an electric field
C) carries a positive charge
D) is a product of natural radioactive decay
E) Two of the above are correct.
A) is more penetrating than a gamma ray
B) is attracted to the positively charged plate in an electric field
C) carries a positive charge
D) is a product of natural radioactive decay
E) Two of the above are correct.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
58
The EPA warns that 8 million homes in the U.S. may have radon contamination higher than the "action level". Why is radon a concern?
A) It is linked to breast cancer.
B) It is linked to lung cancer.
C) It is linked to prostrate cancer.
D) all of these
A) It is linked to breast cancer.
B) It is linked to lung cancer.
C) It is linked to prostrate cancer.
D) all of these

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
59
Which type of radiation has no charge or mass?
A) positron
B) beta
C) alpha
D) gamma
A) positron
B) beta
C) alpha
D) gamma

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
60
What is the process in which neutrons from one fission reaction can cause multiple fission reactions in nearby nuclei?
A) critical mass
B) radiocarbon dating
C) chain reaction
D) binding energy
A) critical mass
B) radiocarbon dating
C) chain reaction
D) binding energy

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
61
The mass numbers of stable isotopes are always twice as large (or larger) than the atomic number.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
62
Elements with an atomic number greater than 83 are unstable.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
63
The following represents a correct conversion factor.
1 Ci = 106 dps
1 Ci = 106 dps

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
64
Consider the following graph. 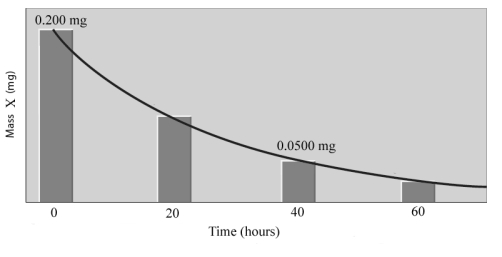 Answer the following questions based on this graph.
Answer the following questions based on this graph.
After 20 hours, ______mg of sample X will remain.
 Answer the following questions based on this graph.
Answer the following questions based on this graph.After 20 hours, ______mg of sample X will remain.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
65
emission occurs in isotopes that have too many neutrons to be stable..

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
66
Consider the following graph.  Answer the following questions based on this graph.
Answer the following questions based on this graph.
After 60 hours, ______mg of sample X will remain.
 Answer the following questions based on this graph.
Answer the following questions based on this graph.After 60 hours, ______mg of sample X will remain.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
67
After three half-lives, 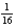 of the original radioactive sample remains.
of the original radioactive sample remains.
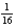 of the original radioactive sample remains.
of the original radioactive sample remains.
Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
68
A transuranium element is a naturally occurring element with more the 92 neutrons.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
69
Beta particles, positrons and gamma rays all are considered to have a mass of 0.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
70
Consider the following graph.  Answer the following questions based on this graph.
Answer the following questions based on this graph.
At 80 hours, _____half-lives will have occurred.
 Answer the following questions based on this graph.
Answer the following questions based on this graph.At 80 hours, _____half-lives will have occurred.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
71
Consider the following graph.  Answer the following questions based on this graph.
Answer the following questions based on this graph.
If the 750 mg sample of element X had been used, the half-life would be ______hours.
 Answer the following questions based on this graph.
Answer the following questions based on this graph.If the 750 mg sample of element X had been used, the half-life would be ______hours.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
72
Consider the following graph.  Answer the following questions based on this graph.
Answer the following questions based on this graph.
If a 500. mg sample of element X had been used, _____mg would remain after 60 hours.
 Answer the following questions based on this graph.
Answer the following questions based on this graph.If a 500. mg sample of element X had been used, _____mg would remain after 60 hours.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
73
Consider the following graph.  Answer the following questions based on this graph.
Answer the following questions based on this graph.
The half-life of element X is _____hours.
 Answer the following questions based on this graph.
Answer the following questions based on this graph.The half-life of element X is _____hours.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck



