Deck 18: The Chemistry of Useful Materials
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/65
Play
Full screen (f)
Deck 18: The Chemistry of Useful Materials
1
What structural unit is characteristic of quartz and most silicates?
A) planar
B) trigonal planar
C) tetrahedral
D) linear
A) planar
B) trigonal planar
C) tetrahedral
D) linear
tetrahedral
2
Which of the following does not apply to silicates?
A) clay
B) tetrahedral units
C) contain carbon
D) SiO2
A) clay
B) tetrahedral units
C) contain carbon
D) SiO2
contain carbon
3
In the reduction of iron in a blast furnace, calcium carbonate, from limestone, is added to combine with what impurity in the iron ore?
A) other metals
B) carbon
C) silicon dioxide
D) sulfur
A) other metals
B) carbon
C) silicon dioxide
D) sulfur
silicon dioxide
4
The chloralkali process uses electrolysis of aqueous sodium chloride to produce all but which one of the following?
A) Cl2
B) NaOH
C) H2
D) CO2
A) Cl2
B) NaOH
C) H2
D) CO2

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
5
Which form of iron contains the least amount of carbon?
A) steel
B) pig iron
C) cast iron
D) These forms of iron contain no carbon.
A) steel
B) pig iron
C) cast iron
D) These forms of iron contain no carbon.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
6
Which kind of rocks are formed by solidification of molten rock?
A) igneous
B) metamorphic
C) sedimentary
D) both b and c
A) igneous
B) metamorphic
C) sedimentary
D) both b and c

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
7
The process by which copper is prepared from its ores is
A) electrolysis.
B) roasting.
C) the chloralkali process.
D) both electrolysis and roasting
A) electrolysis.
B) roasting.
C) the chloralkali process.
D) both electrolysis and roasting

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
8
Pyrex and Kimax glass typically contain an oxide of which element that gives the glass a small coefficient of thermal expansion?
A) sodium
B) lead
C) boron
D) arsenic
A) sodium
B) lead
C) boron
D) arsenic

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
9
The most abundant substances in rocks are
A) sulfates.
B) carbonates.
C) silicates.
D) oxides.
A) sulfates.
B) carbonates.
C) silicates.
D) oxides.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following elements comprises more the 25% of both the Earth's crust and the whole Earth?
A) hydrogen
B) oxygen
C) iron
D) aluminum
A) hydrogen
B) oxygen
C) iron
D) aluminum

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is the most abundant metal in the Earth's crust?
A) magnesium, Mg
B) iron, Fe
C) nickel, Ni
D) aluminum, Al
A) magnesium, Mg
B) iron, Fe
C) nickel, Ni
D) aluminum, Al

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
12
Which metal is sufficiently concentrated in the oceans so that it is currently extracted commercially?
A) aluminum
B) copper
C) magnesium
D) iron
A) aluminum
B) copper
C) magnesium
D) iron

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
13
Which mineral is likely to be used in cement and wallboard?
A) oxides
B) silicates
C) sulfates
D) Oxides
A) oxides
B) silicates
C) sulfates
D) Oxides

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
14
Which substance is formed when silica is melted (so that SiO4 units are reorganized) and then cooled?
A) clay
B) glass
C) talc
D) feldspar
A) clay
B) glass
C) talc
D) feldspar

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following metals and ores is not a correct pair?
A) zinc, ZnS
B) iron, Fe2O3
C) gold, AuO
D) lead, PbS
A) zinc, ZnS
B) iron, Fe2O3
C) gold, AuO
D) lead, PbS

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
16
Over the years what element has been used as a reducing agent to reduce iron metal from its ores?
A) sulfur
B) silicon
C) sodium
D) carbon
A) sulfur
B) silicon
C) sodium
D) carbon

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
17
Older glass beverage bottles were green due to the presence of which metal ion in the silica from which the bottle was made?
A) Fe2+
B) Cu2+
C) Sn4+
D) Ca2+
A) Fe2+
B) Cu2+
C) Sn4+
D) Ca2+

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is not a property of ceramics?
A) ability to withstand high temperatures
B) conductivity
C) tendency to fail in response to stress
D) cheap raw materials
A) ability to withstand high temperatures
B) conductivity
C) tendency to fail in response to stress
D) cheap raw materials

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following metals is recovered from sea water?
A) magnesium
B) iron
C) copper
D) both b and c
A) magnesium
B) iron
C) copper
D) both b and c

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
20
Which term or phrase does not apply to steel?
A) used in structural support of buildings
B) made in basic oxygen process
C) can be magnetic
D) has a high carbon content
A) used in structural support of buildings
B) made in basic oxygen process
C) can be magnetic
D) has a high carbon content

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
21
Which commonly used structural metal has the lowest density?
A) aluminum
B) zinc
C) magnesium
D) titanium
A) aluminum
B) zinc
C) magnesium
D) titanium

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
22
Amorphous materials
A) are solids like tar and glass that lack internal order.
B) are solids with regular order.
C) have a definite crystal structure.
D) are crystalline solids.
A) are solids like tar and glass that lack internal order.
B) are solids with regular order.
C) have a definite crystal structure.
D) are crystalline solids.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following statements characterizes clays?
A) chain-like structure
B) contains silicon and iron
C) similar to silica in structure
D) component of soil
E) all of these
A) chain-like structure
B) contains silicon and iron
C) similar to silica in structure
D) component of soil
E) all of these

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
24
To produce steel in the blast furnace, what must done to the carbon content in the iron?
A) increased to approximately twice its original value
B) reduced to a smaller value
C) eliminated
D) distributed evenly throughout the iron
A) increased to approximately twice its original value
B) reduced to a smaller value
C) eliminated
D) distributed evenly throughout the iron

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
25
Solar cells can be
A) built only using layers p-type of transistors.
B) built only using layers n-type of transistors.
C) built using alternate layers of n-type and p-type transistors.
D) rechargeable.
A) built only using layers p-type of transistors.
B) built only using layers n-type of transistors.
C) built using alternate layers of n-type and p-type transistors.
D) rechargeable.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
26
A p-type semiconductor is one that
A) contains more electrons than silicon.
B) is deficient electrons.
C) has no electrons.
D) has the same number of electrons as silicon.
A) contains more electrons than silicon.
B) is deficient electrons.
C) has no electrons.
D) has the same number of electrons as silicon.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
27
The p-type of semiconductor is generally
A) silicon doped with a Group 5 element.
B) silicon doped with a Group 14 element.
C) germanium doped with an element from Group 14.
D) silicon doped with an element from Group 13.
A) silicon doped with a Group 5 element.
B) silicon doped with a Group 14 element.
C) germanium doped with an element from Group 14.
D) silicon doped with an element from Group 13.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following metals is reduced by an electron flow in an electric furnace rather than by chemical reduction?
A) iron
B) copper
C) lead
D) aluminum
A) iron
B) copper
C) lead
D) aluminum

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
29
Calcium oxide from oyster shells is used to precipitate magnesium from sea water. The oxide reacts with water to form
A) hydrogen.
B) a sulfate.
C) a hydroxide.
D) oxide ions which precipitate the magnesium in the aqueous medium.
A) hydrogen.
B) a sulfate.
C) a hydroxide.
D) oxide ions which precipitate the magnesium in the aqueous medium.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
30
Doped silicon is used in
A) magnets.
B) transistors.
C) electrical transmission wires.
D) superconductors.
A) magnets.
B) transistors.
C) electrical transmission wires.
D) superconductors.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
31
Most metals are found in the Earth as
A) positive ions.
B) negative ions.
C) neutral atoms.
D) molecules.
A) positive ions.
B) negative ions.
C) neutral atoms.
D) molecules.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following elements is found in the Earth's crust as well as in the mantle and inner and outer cores?
A) aluminum
B) oxygen
C) silicon
D) iron
A) aluminum
B) oxygen
C) silicon
D) iron

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
33
Roasting of metal ores in air
A) converts sulfides to oxides.
B) converts sulfides to pure metals.
C) converts oxides to silicates.
D) reduces metal oxides to sulfides.
A) converts sulfides to oxides.
B) converts sulfides to pure metals.
C) converts oxides to silicates.
D) reduces metal oxides to sulfides.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
34
Glass is annealed so that
A) silicon is baked out of the mixture.
B) calcium carbonate is decomposed to form calcium oxide.
C) strain is minimized as the liquid cools.
D) none of these
A) silicon is baked out of the mixture.
B) calcium carbonate is decomposed to form calcium oxide.
C) strain is minimized as the liquid cools.
D) none of these

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following is not an ingredient in Portland cement?
A) anhydrous calcium oxide
B) silicon dioxide
C) hydrated calcium sulfate
D) aluminum oxide
A) anhydrous calcium oxide
B) silicon dioxide
C) hydrated calcium sulfate
D) aluminum oxide

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
36
Asbestos is
A) a glass.
B) a form of silicate.
C) amorphous.
D) a form of clay.
A) a glass.
B) a form of silicate.
C) amorphous.
D) a form of clay.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
37
Superconductivity is the result of
A) extremely low electrical resistance.
B) increases in available energy.
C) very high concentrations of electrical charge.
D) higher levels achieved in the purity of copper.
A) extremely low electrical resistance.
B) increases in available energy.
C) very high concentrations of electrical charge.
D) higher levels achieved in the purity of copper.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following can be melted with silica (silicon dioxide) to form common window glass?
A) carbon
B) carbonates of sodium and calcium
C) sodium sulfate and sodium sulfite
D) sand containing small amounts of magnesium
A) carbon
B) carbonates of sodium and calcium
C) sodium sulfate and sodium sulfite
D) sand containing small amounts of magnesium

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
39
A common element used in many semiconductors is
A) silicon.
B) sodium.
C) silver.
D) carbon.
A) silicon.
B) sodium.
C) silver.
D) carbon.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
40
Bauxite is the name of an aluminum containing mineral. Which of the following does not describe a mineral?
A) naturally occurring
B) inorganic
C) solid
D) all of these
A) naturally occurring
B) inorganic
C) solid
D) all of these

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following is an example of metamorphic rock?
A) basalt
B) sandstone
C) marble
D) sand
A) basalt
B) sandstone
C) marble
D) sand

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
42
The first transistor was constructed
A) in 1947 at Bell Laboratories.
B) in 1941 at IBM.
C) in 1969 at Texas Instruments.
D) in 1934 in Switzerland.
A) in 1947 at Bell Laboratories.
B) in 1941 at IBM.
C) in 1969 at Texas Instruments.
D) in 1934 in Switzerland.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
43
Examine the chloralkali cell given below. 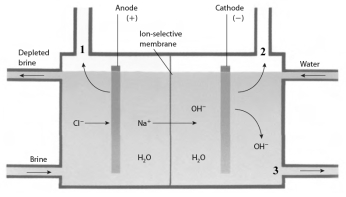 Answer the following questions with the identity of the chemical substance indicated.
Answer the following questions with the identity of the chemical substance indicated.
The product of the cell reaction produced at the position indicated by the number 3 is ______________.
 Answer the following questions with the identity of the chemical substance indicated.
Answer the following questions with the identity of the chemical substance indicated.The product of the cell reaction produced at the position indicated by the number 3 is ______________.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
44
Examine the chloralkali cell given below.  Answer the following questions with the identity of the chemical substance indicated.
Answer the following questions with the identity of the chemical substance indicated.
The compound that is used as the reactant in the cell, indicated by the term brine, is ______________.
 Answer the following questions with the identity of the chemical substance indicated.
Answer the following questions with the identity of the chemical substance indicated.The compound that is used as the reactant in the cell, indicated by the term brine, is ______________.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
45
Examine the chloralkali cell given below.  Answer the following questions with the identity of the chemical substance indicated.
Answer the following questions with the identity of the chemical substance indicated.
The product of the cell reaction produced at the position indicated by the number 1 is ______________.
 Answer the following questions with the identity of the chemical substance indicated.
Answer the following questions with the identity of the chemical substance indicated.The product of the cell reaction produced at the position indicated by the number 1 is ______________.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
46
Arsenic, As, which has five valence electrons can be used in doping to product an n-type semiconductor.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
47
Examine the chloralkali cell given below.  Answer the following questions with the identity of the chemical substance indicated.
Answer the following questions with the identity of the chemical substance indicated.
The product of the cell reaction produced at the position indicated by the number 2 is ______________.
 Answer the following questions with the identity of the chemical substance indicated.
Answer the following questions with the identity of the chemical substance indicated.The product of the cell reaction produced at the position indicated by the number 2 is ______________.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
48
Silver is likely to be found in nature as the free element while lead and aluminum are found in compounds.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
49
Consider the diagram of the blast furnace given below. 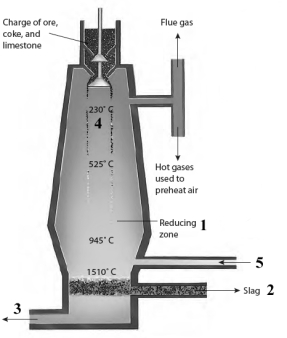 Answer the following questions with the numbers shown in the image.
Answer the following questions with the numbers shown in the image.
Iron ions form iron atoms in the area indicated by the number ______.
 Answer the following questions with the numbers shown in the image.
Answer the following questions with the numbers shown in the image.Iron ions form iron atoms in the area indicated by the number ______.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
50
What two elements make up glass?
A) Si and S
B) Si and B
C) Si and N
D) Si and O
A) Si and S
B) Si and B
C) Si and N
D) Si and O

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
51
Most of the material in the Earth's crust is composed of a homogenous collection of various compounds.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
52
Consider the following image.  The model shown in the image represents the bonding found in
The model shown in the image represents the bonding found in
A) metals
B) superconductors
C) n-type semiconductors
D) p-type semiconductors
 The model shown in the image represents the bonding found in
The model shown in the image represents the bonding found inA) metals
B) superconductors
C) n-type semiconductors
D) p-type semiconductors

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
53
How many valence electrons would be found in an atom used as dopant in a p-type semiconductor?
A) 3
B) 4
C) 5
D) 6
A) 3
B) 4
C) 5
D) 6

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
54
Pig iron is formed in a blast furnace.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
55
The major component of sea shells is calcium silicate.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
56
Consider the diagram of the blast furnace given below.  Answer the following questions with the numbers shown in the image.
Answer the following questions with the numbers shown in the image.
Silica from the sand used in the furnace is removed in the area indicated by the number ______.
 Answer the following questions with the numbers shown in the image.
Answer the following questions with the numbers shown in the image.Silica from the sand used in the furnace is removed in the area indicated by the number ______.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
57
Which material is structurally stronger?
A) pig iron
B) cast iron
C) steel
D) iron oxide
A) pig iron
B) cast iron
C) steel
D) iron oxide

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
58
To make an n-type semiconductor, silicon is doped with which of the following elements?
A) germanium
B) arsenic
C) boron
D) magnesium
A) germanium
B) arsenic
C) boron
D) magnesium

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following is a property of a metal?
A) ductile and malleable
B) low electrical conductivity
C) low thermal conductivity
D) soluble in water
A) ductile and malleable
B) low electrical conductivity
C) low thermal conductivity
D) soluble in water

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
60
The main source of the metal magnesium, Mg, is the Earth's crust.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
61
Consider the diagram shown below which represents from left to right a pure silicon crystal and two silicon crystals containing dopants. The larger dots in the image are the valence electrons of A and B. 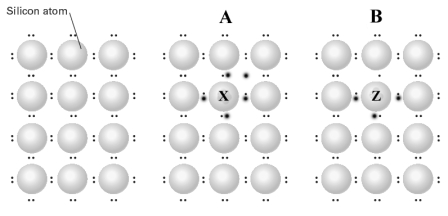 Complete the following statements using the letters in the diagram.
Complete the following statements using the letters in the diagram.
A p-type semiconductor is labeled with the letter______.
 Complete the following statements using the letters in the diagram.
Complete the following statements using the letters in the diagram.A p-type semiconductor is labeled with the letter______.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
62
Consider the diagram shown below which represents from left to right a pure silicon crystal and two silicon crystals containing dopants. The larger dots in the image are the valence electrons of A and B.  Complete the following statements using the letters in the diagram.
Complete the following statements using the letters in the diagram.
An n-type semiconductor is labeled with the letter______.
 Complete the following statements using the letters in the diagram.
Complete the following statements using the letters in the diagram.An n-type semiconductor is labeled with the letter______.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
63
Consider the diagram shown below which represents from left to right a pure silicon crystal and two silicon crystals containing dopants. The larger dots in the image are the valence electrons of A and B.  Complete the following statements using the letters in the diagram.
Complete the following statements using the letters in the diagram.
Selenium could be used as the dopant indicated by the letter______.
 Complete the following statements using the letters in the diagram.
Complete the following statements using the letters in the diagram.Selenium could be used as the dopant indicated by the letter______.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
64
Consider the diagram shown below which represents from left to right a pure silicon crystal and two silicon crystals containing dopants. The larger dots in the image are the valence electrons of A and B.  Complete the following statements using the letters in the diagram.
Complete the following statements using the letters in the diagram.
Germanium could be used as the dopant indicated by the letter ______.
 Complete the following statements using the letters in the diagram.
Complete the following statements using the letters in the diagram.Germanium could be used as the dopant indicated by the letter ______.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
65
Consider the diagram of the blast furnace given below.  Answer the following questions with the numbers shown in the image.
Answer the following questions with the numbers shown in the image.
The area in which pig iron would be found is indicated by the number ______.
 Answer the following questions with the numbers shown in the image.
Answer the following questions with the numbers shown in the image.The area in which pig iron would be found is indicated by the number ______.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck



