Deck 28: Carbohydrates
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/41
Play
Full screen (f)
Deck 28: Carbohydrates
1
Which of the following is a reducing sugar? 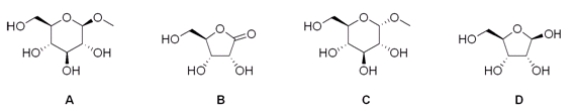
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D
D
2
Which of the following compounds is an aldose? 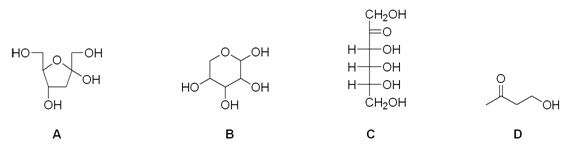
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D
B
3
Which of the following reagents would convert glucose into ethyl glucopyranoside?
A) EtBr, aq. HCl
B) EtOH, aq. HCl
C) EtOH, NaOH
D) Ag2O in NH4OH
A) EtBr, aq. HCl
B) EtOH, aq. HCl
C) EtOH, NaOH
D) Ag2O in NH4OH
EtOH, aq. HCl
4
Amylose is a polysaccharide with ________________________ linkages.
A) " -1,4'-glycosidic"
B) " -1,4'-glycosidic"
C) " -1,6'-glycosidic"
D) " -1,6'-glycosidic"
A) " -1,4'-glycosidic"
B) " -1,4'-glycosidic"
C) " -1,6'-glycosidic"
D) " -1,6'-glycosidic"

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
5
How many carbons does a pentose sugar have?
A) 2
B) 3
C) 4
D) 5
A) 2
B) 3
C) 4
D) 5

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following compounds is a anomer? 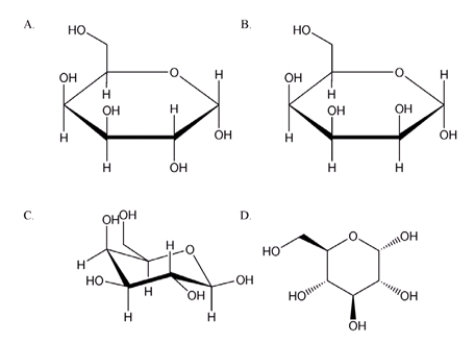
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
7
Which is the correct Haworth projection for the anomer of D-glucose? 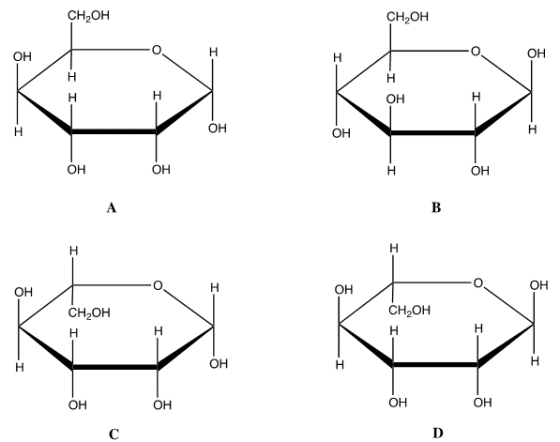
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
8
What is the correct chair form for the anomer of D-glucose? 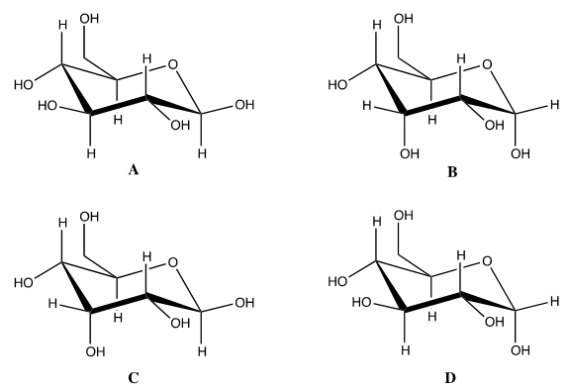
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
9
Does a monosaccharide prefer to exist in the open-chain or closed-ring form?
A) open
B) closed
C) Both forms exist in equal ratios.
A) open
B) closed
C) Both forms exist in equal ratios.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
10
Which is the correct Haworth projection for the anomer of allose? 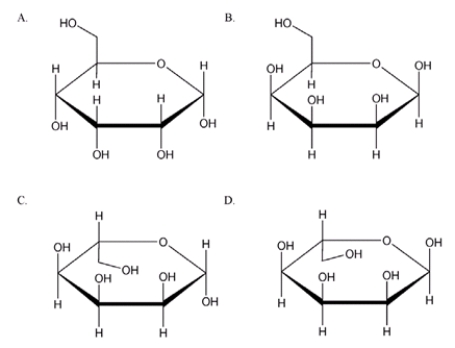
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following compounds is not a valid representation for D-glucose? 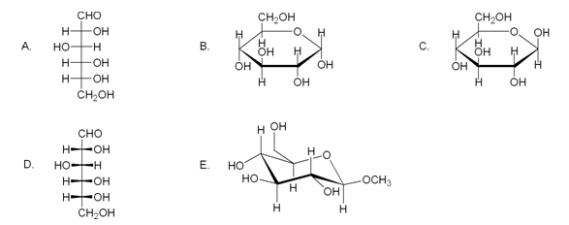
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
12
What are the products of the following reaction? 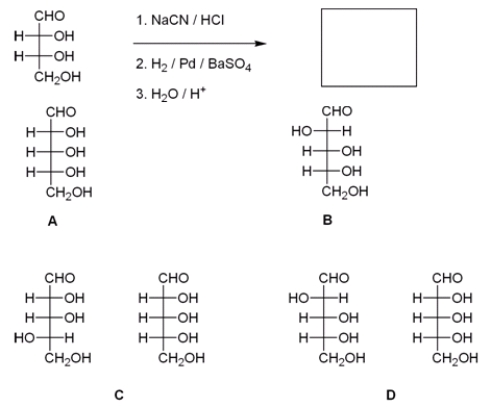
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
13
Which is the correct Fischer projection for the open-chain form of the carbohydrate shown below? 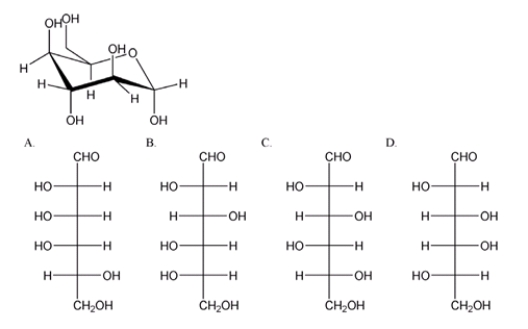
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
14
What is the process called for the interconversion of one anomer to the other?
A) racemization
B) mutarotation
C) epimerization
D) enolization
A) racemization
B) mutarotation
C) epimerization
D) enolization

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following pairs of carbohydrates will afford the same product from a Wohl degradation?
A) xylose/glucose
B) ribose/arabinose
C) fructose/mannose
D) lyxose/threose
A) xylose/glucose
B) ribose/arabinose
C) fructose/mannose
D) lyxose/threose

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
16
Is the following carbohydrate D or L? Provide an explanation for your answer. 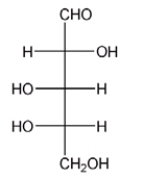
A) It is D because the OH group on C2 is on the right.
B) It is L because the OH group on C4 is on the left.
C) It is L because the OH group on C3 is on the left.
D) It is D because the sugar is not symmetric.

A) It is D because the OH group on C2 is on the right.
B) It is L because the OH group on C4 is on the left.
C) It is L because the OH group on C3 is on the left.
D) It is D because the sugar is not symmetric.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
17
How many possible stereoisomers are there of the following heptose? 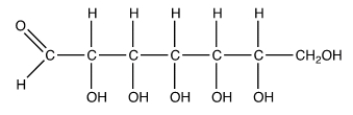
A) 16
B) 21
C) 32
D) 64

A) 16
B) 21
C) 32
D) 64

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
18
What is the correct chair form for the anomer of D-mannose? 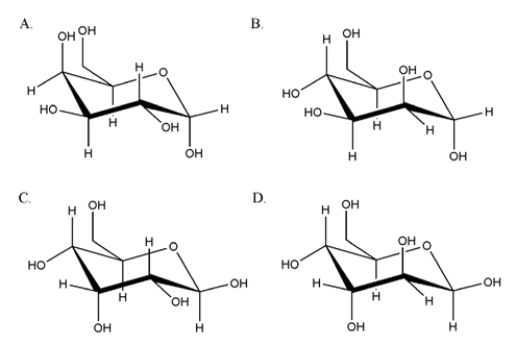
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following reagents will selectively oxidize an aldose to an aldonic acid?
A) PCC
B) Ag2O in NH4OH
C) FeCl3
D) HNO3
A) PCC
B) Ag2O in NH4OH
C) FeCl3
D) HNO3

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
20
Cellulose is a polysaccharide with ________________________ linkages.
A) " -1,4'-glycosidic"
B) " -1,4'-glycosidic"
C) " -1,6'-glycosidic"
D) " -1,6'-glycosidic"
A) " -1,4'-glycosidic"
B) " -1,4'-glycosidic"
C) " -1,6'-glycosidic"
D) " -1,6'-glycosidic"

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
21
From the sugars shown below, which one is a non-reducing sugar? 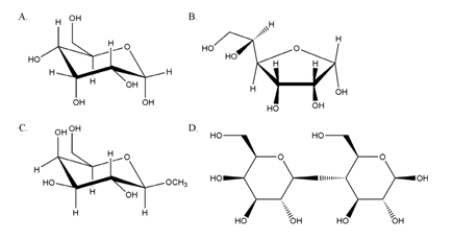
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
22
Upon hydrolysis of cellulose, what sugar is formed?
A) sucrose
B) maltose
C) lactose
D) glucose
A) sucrose
B) maltose
C) lactose
D) glucose

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
23
Upon hydrolysis of amylose, what sugar is formed?
A) sucrose
B) maltose
C) lactose
D) glucose
A) sucrose
B) maltose
C) lactose
D) glucose

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
24
The branching units in amylopectin, a polysaccharide, which enable this molecule to be water soluble are connected with __________________________ linkages.
A) " -1,4'-glycosidic"
B) " -1,4'-glycosidic"
C) " -1,6'-glycosidic"
D) " -1,6'-glycosidic"
A) " -1,4'-glycosidic"
B) " -1,4'-glycosidic"
C) " -1,6'-glycosidic"
D) " -1,6'-glycosidic"

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
25
From the disaccharides listed below, which one will not undergo mutarotation?
A) sucrose
B) maltose
C) lactose
D) xylobiose
A) sucrose
B) maltose
C) lactose
D) xylobiose

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
26
After a series of Kiliani-Fischer syntheses on D-glyceraldehyde, an unknown sugar was isolated from the reaction mixture. The following experimental data was obtained. What is the structure of this unknown sugar? Data:
- Molecular formula: C6H12O6
- Reacts with phenylhydrazine to give an osazone, mp 178°C.
- Reacts with HNO3 to give an optically active aldaric acid.
- Wohl degradation followed by HNO3 oxidation gives an optically inactive
Aldaric acid.
- Two Wohl degradations followed by HNO3 oxidation give a meso-tartaric acid.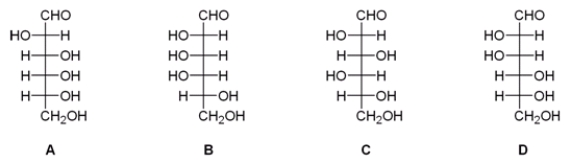
A) A
B) B
C) C
D) D
- Molecular formula: C6H12O6
- Reacts with phenylhydrazine to give an osazone, mp 178°C.
- Reacts with HNO3 to give an optically active aldaric acid.
- Wohl degradation followed by HNO3 oxidation gives an optically inactive
Aldaric acid.
- Two Wohl degradations followed by HNO3 oxidation give a meso-tartaric acid.

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
27
From the sugars shown below, which one will not give a positive test with Tollens reagent? 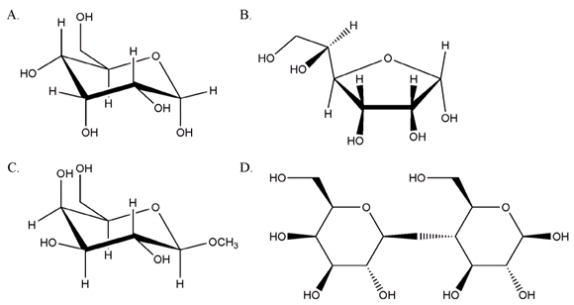
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following sugars can be classified as a ketopentose? 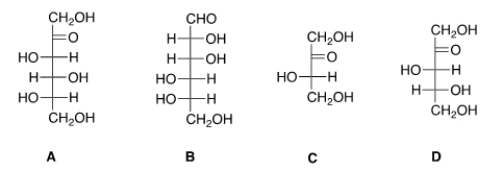
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following best describes the relationship between D-glucose and D-fructose?
A) enantiomers
B) epimers
C) anomers
D) constitutional isomers
A) enantiomers
B) epimers
C) anomers
D) constitutional isomers

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
30
What is the best description of the product formed in the following reaction sequence? 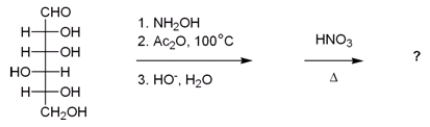
A) The product is an optically active aldonic acid.
B) The product is an optically inactive aldonic acid.
C) The product is an optically active aldaric acid.
D) The product is an optically inactive aldaric acid.

A) The product is an optically active aldonic acid.
B) The product is an optically inactive aldonic acid.
C) The product is an optically active aldaric acid.
D) The product is an optically inactive aldaric acid.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
31
The following reaction is an example of a base-catalyzed epimerization reaction in which a ketose is converted to a pair of aldoses. What are the products from this reaction? 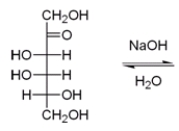
A) D-glucose and D-mannose
B) D-galactose and D-talose
C) D-talose and D-idose
D) D-glucose and D-idose

A) D-glucose and D-mannose
B) D-galactose and D-talose
C) D-talose and D-idose
D) D-glucose and D-idose

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
32
Oxidation of a D-hexose with nitric acid forms an optically active aldaric acid. A Wohl degradation of the same D-hexose followed by oxidation with nitric acid forms an optically active aldaric acid. What are the three possible D-hexoses that fit this data?
A) D-allose, D-altrose and D-glucose
B) D-glucose, D- mannose and D-talose
C) D-glucose, D-idose and D-altrose
D) D-galactose, D-talose and D-mannose
A) D-allose, D-altrose and D-glucose
B) D-glucose, D- mannose and D-talose
C) D-glucose, D-idose and D-altrose
D) D-galactose, D-talose and D-mannose

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
33
Which sugar is represented by the line angle structure below? 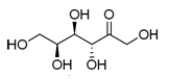
A) D-fructose
B) D-sorbose
C) L-psicose
D) L-tagatose

A) D-fructose
B) D-sorbose
C) L-psicose
D) L-tagatose

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
34
What is the composition of sucrose?
A) two glucose units with a -1,4'-glycoside bond
B) one glucose and one fructose unit with a -glycosidic bond to the 2'carbon of a fructofuranose ring
C) one galactose and one glucose unit with a -1,4'-glycoside bond
D) repeating unit of glucose units joined in a -1,4'-glycosidic linkage
A) two glucose units with a -1,4'-glycoside bond
B) one glucose and one fructose unit with a -glycosidic bond to the 2'carbon of a fructofuranose ring
C) one galactose and one glucose unit with a -1,4'-glycoside bond
D) repeating unit of glucose units joined in a -1,4'-glycosidic linkage

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
35
What is the composition of maltose?
A) two glucose units with a -1,4'-glycoside bond
B) one glucose and one fructose unit with a -glycosidic bond to the 2'carbon of a fructofuranose ring
C) one galactose and one glucose unit with a -1,4'-glycoside bond
D) repeating unit of glucose units joined in a -1,4'-glycosidic linkage
A) two glucose units with a -1,4'-glycoside bond
B) one glucose and one fructose unit with a -glycosidic bond to the 2'carbon of a fructofuranose ring
C) one galactose and one glucose unit with a -1,4'-glycoside bond
D) repeating unit of glucose units joined in a -1,4'-glycosidic linkage

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
36
Upon hydrolysis of amylopectin, what sugar is formed?
A) sucrose
B) maltose
C) lactose
D) glucose
A) sucrose
B) maltose
C) lactose
D) glucose

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following sugars can be classified as a ketohexose? 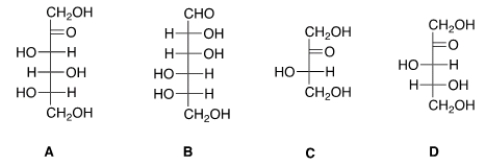
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
38
What is the composition of lactose?
A) two glucose units with a -1,4'-glycoside bond
B) one glucose and one fructose unit with a -glycosidic bond to the 2'carbon of a fructofuranose ring
C) one galactose and one glucose unit with a -1,4'-glycoside bond
D) repeating unit of glucose units joined in a -1,4'-glycosidic linkage
A) two glucose units with a -1,4'-glycoside bond
B) one glucose and one fructose unit with a -glycosidic bond to the 2'carbon of a fructofuranose ring
C) one galactose and one glucose unit with a -1,4'-glycoside bond
D) repeating unit of glucose units joined in a -1,4'-glycosidic linkage

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following sugars can be classified as a ketotetrose? 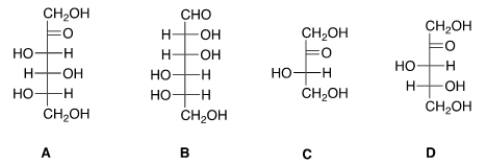
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following sugars can be classified as an aldohexose? 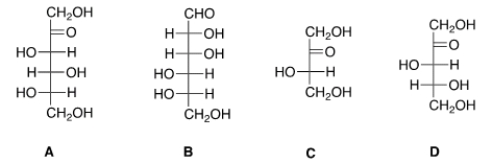
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
41
What is the classification of pyranose forms of monosaccharides?
A) 5-membered cyclic hemiacetals
B) 6-membered cyclic acetals
C) 5-membered cyclic acetals
D) 6-membered cyclic hemiacetals
A) 5-membered cyclic hemiacetals
B) 6-membered cyclic acetals
C) 5-membered cyclic acetals
D) 6-membered cyclic hemiacetals

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck



