Deck 22: Carboxylic Acids and Derivatives
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/47
Play
Full screen (f)
Deck 22: Carboxylic Acids and Derivatives
1
Which of the following peaks would you see in the IR spectrum of a carboxylic acid?
A) A flat line (carboxylic acids are not IR active)
B) A sharp line at 2250 cm-1
C) A broad peak from 3800-2800 cm-1
D) A broad peak from 800-600 cm-1
A) A flat line (carboxylic acids are not IR active)
B) A sharp line at 2250 cm-1
C) A broad peak from 3800-2800 cm-1
D) A broad peak from 800-600 cm-1
A broad peak from 3800-2800 cm-1
2
Where is the carbonyl absorption found in the IR spectrum of a simple ester?
A) 2.5 ppm
B) 2250 cm-1
C) 3800 cm-1
D) 1740 cm-1
A) 2.5 ppm
B) 2250 cm-1
C) 3800 cm-1
D) 1740 cm-1
1740 cm-1
3
What is the IUPAC name for the following compound? 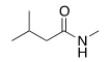
A) methyl 3-methylbutanamide
B) 5-methylhexanamide
C) N-methyl 3-methylhexanamide
D) N-methyl 3-methylbutanamide

A) methyl 3-methylbutanamide
B) 5-methylhexanamide
C) N-methyl 3-methylhexanamide
D) N-methyl 3-methylbutanamide
N-methyl 3-methylbutanamide
4
Why is an amide less reactive to nucleophilic acyl substitution than an acid chloride?
A) Nitrogen is a better leaving group.
B) Chloride is a better leaving group.
C) Nitrogen donates more electron density into the carbonyl.
D) The amide anion is less basic.
A) Nitrogen is a better leaving group.
B) Chloride is a better leaving group.
C) Nitrogen donates more electron density into the carbonyl.
D) The amide anion is less basic.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
5
What is the IUPAC name for the following compound? 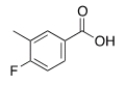
A) 4-fluoro-3-methylbenzoate
B) 4-fluoro-3-methylbenzoic acid
C) 3-methyl-4-fluorobenzoic acid
D) 4-fluoro-5-methylbenzoic acid

A) 4-fluoro-3-methylbenzoate
B) 4-fluoro-3-methylbenzoic acid
C) 3-methyl-4-fluorobenzoic acid
D) 4-fluoro-5-methylbenzoic acid

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
6
Will the following reaction occur? 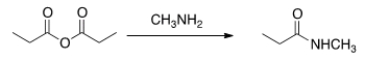
A) Yes
B) No

A) Yes
B) No

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
7
Why doesn't nucleophilic acyl substitution stop at the tetrahedral intermediate?
A) The nucleophile is too basic.
B) Reforming the carbonyl is energetically favorable.
C) The leaving group is unstable and wants to be negatively charged.
D) There is no tetrahedral intermediate.
A) The nucleophile is too basic.
B) Reforming the carbonyl is energetically favorable.
C) The leaving group is unstable and wants to be negatively charged.
D) There is no tetrahedral intermediate.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
8
Where do the carbonyl signals appear in the 13C NMR spectrum of carboxylic acid derivatives?
A) 1700 cm-1
B) 180-160 ppm
C) 2.5-3.0 ppm
D) 100-80 ppm
A) 1700 cm-1
B) 180-160 ppm
C) 2.5-3.0 ppm
D) 100-80 ppm

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
9
Why is pyridine included in the reaction of an acid chloride and an amine or alcohol?
A) Pyridine will deprotonate the amine or alcohol, making it a better nucleophile.
B) Pyridine will neutralize the acid by-product of the reaction.
C) Pyridine will protonate the carbonyl of the acid chloride, making it more reactive.
D) Pyridine will absorb the heat of the reaction.
A) Pyridine will deprotonate the amine or alcohol, making it a better nucleophile.
B) Pyridine will neutralize the acid by-product of the reaction.
C) Pyridine will protonate the carbonyl of the acid chloride, making it more reactive.
D) Pyridine will absorb the heat of the reaction.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
10
You see an absorption at 2250 cm-1 in the IR spectrum of a compound. What kind of functional group is present?
A) A ketone
B) An aldehyde
C) An ester
D) A nitrile
A) A ketone
B) An aldehyde
C) An ester
D) A nitrile

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
11
What is the first step in the general mechanism for nucleophilic acyl substitution?
A) Protonation of the carbonyl
B) Removal of an -proton
C) Addition of the nucleophile to the carbonyl
D) Loss of the leaving group
A) Protonation of the carbonyl
B) Removal of an -proton
C) Addition of the nucleophile to the carbonyl
D) Loss of the leaving group

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
12
What is the structure for ethyl 4-chlorobenzoate? 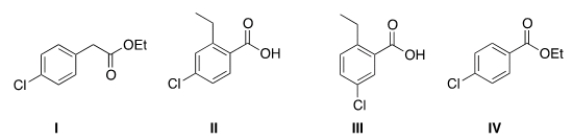
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
13
What is the IUPAC name for the following compound? 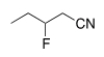
A) 2-fluorobutanonitrile
B) 2-fluoropentanonitrile
C) 3-fluoropentanonitrile
D) 2-fluorobutylcyanide

A) 2-fluorobutanonitrile
B) 2-fluoropentanonitrile
C) 3-fluoropentanonitrile
D) 2-fluorobutylcyanide

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
14
What is the structure for 3-cyanocyclopentanone? 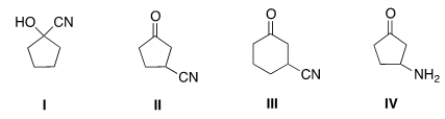
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
15
Will the following reaction occur? 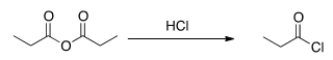
A) Yes
B) No

A) Yes
B) No

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
16
Which structure is consistent with the following 1H NMR spectrum? 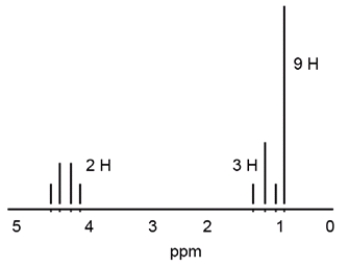
A) ethyl acetate
B) 2-propanone
C) 3,3-dimethyl-2-pentanone
D) ethyl 2,2-dimethylpropionate

A) ethyl acetate
B) 2-propanone
C) 3,3-dimethyl-2-pentanone
D) ethyl 2,2-dimethylpropionate

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
17
What is the structure for acetic propanoic anhydride? 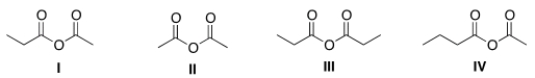
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
18
What is the product of the following reaction? 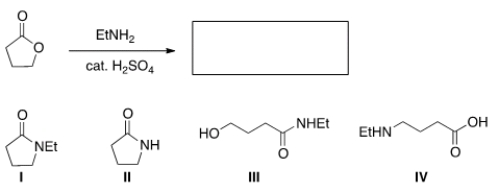
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is a lactam? 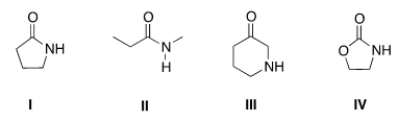
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
20
Will the following reaction occur? 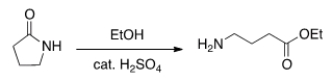
A) Yes
B) No

A) Yes
B) No

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
21
What is the product of the following reaction (after acidic work-up)? 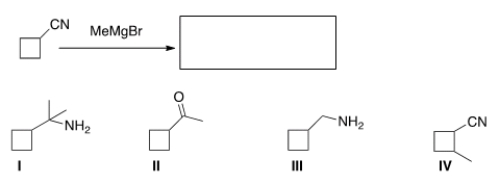
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
22
What is the product of the following reaction? 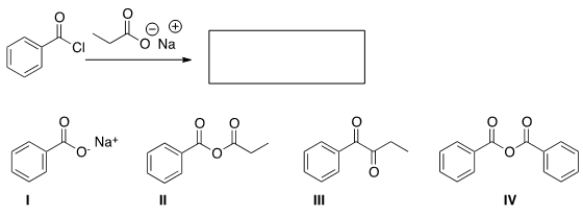
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
23
How can you convert a carboxylic acid into an ester?
A) Heat with an alcohol and catalytic acid
B) Deprotonate with a base and react with an alcohol
C) Deprotonate with a base and react with an alkyl halide
D) Both (a) heat with an alcohol and catalytic acid and (c) deprotonate with a base and react with an alkyl halide
E) Both (a) heat with an alcohol and catalytic acid and (b) deprotonate with a base and react with an alcohol
A) Heat with an alcohol and catalytic acid
B) Deprotonate with a base and react with an alcohol
C) Deprotonate with a base and react with an alkyl halide
D) Both (a) heat with an alcohol and catalytic acid and (c) deprotonate with a base and react with an alkyl halide
E) Both (a) heat with an alcohol and catalytic acid and (b) deprotonate with a base and react with an alcohol

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
24
What is the product of the following reaction? 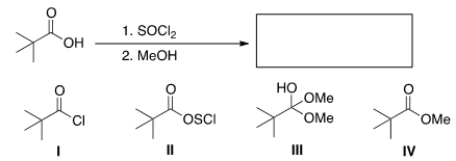
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
25
Rank the following compounds in order of increasing reactivity in nucleophilic acyl substitution. 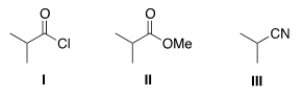
A) I > II > III
B) III > II > I
C) II > III > I
D) III > I > II

A) I > II > III
B) III > II > I
C) II > III > I
D) III > I > II

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
26
What is the direct product of the base-promoted hydrolysis of an ester?
A) A nitrile
B) A carboxylic acid
C) An amide
D) A carboxylic acid salt
A) A nitrile
B) A carboxylic acid
C) An amide
D) A carboxylic acid salt

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following two amides will react more readily with a nucleophile? Why? 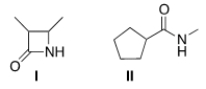
A) I, because it is less hindered
B) II, because it is less hindered
C) I, because it is more strained
D) II, because it is more strained

A) I, because it is less hindered
B) II, because it is less hindered
C) I, because it is more strained
D) II, because it is more strained

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
28
What is the product of the following reaction? 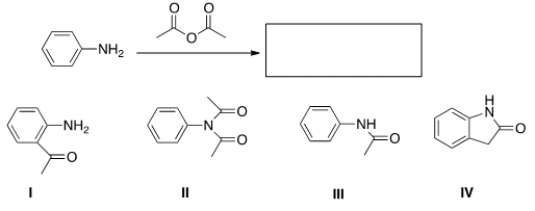
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
29
What is the product of the following reaction? 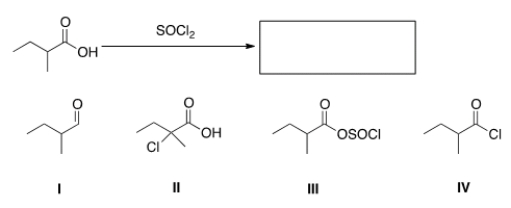
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
30
What is the product of the following reaction? 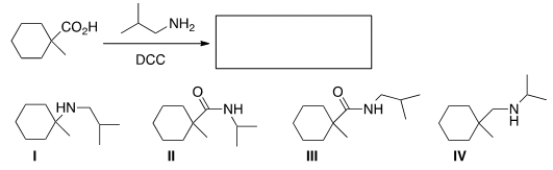
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
31
What is the structure for the compound whose IUPAC name is pentyl 2-methylbutanoate? 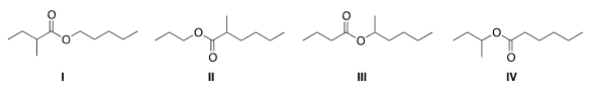
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
32
What reagent would you use for the following transformation? 
A) LiAlH4
B) DIBAL-H
C) NaBH4
D) H2, Pd/C

A) LiAlH4
B) DIBAL-H
C) NaBH4
D) H2, Pd/C

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
33
How can you convert a carboxylic acid into an acid chloride?
A) Heat with hydrochloric acid
B) React with thionyl chloride (SOCl2)
C) React with sodium chloride
D) React with Cl2 and FeCl3
A) Heat with hydrochloric acid
B) React with thionyl chloride (SOCl2)
C) React with sodium chloride
D) React with Cl2 and FeCl3

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
34
What is the product of the following reaction? 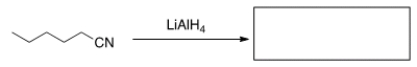
A) Hexanoic acid
B) 1-Hexanamide
C) Hexanal
D) 1-Hexanamine

A) Hexanoic acid
B) 1-Hexanamide
C) Hexanal
D) 1-Hexanamine

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
35
What is the product of the following reaction? 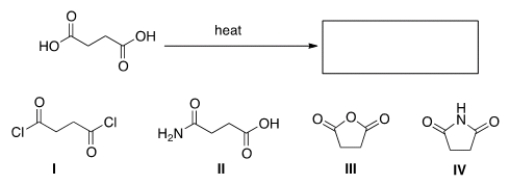
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
36
What is the product of the following reaction? 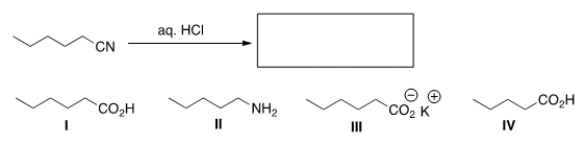
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following reaction conditions can be used to synthesize an ester (RCOOR')?
A) RCOCl + R'NH2 + pyridine
B) RCOOH + R'OH + H+
C) RCOOH + R'OH + OH-
D) RCONH2 + R'OH
A) RCOCl + R'NH2 + pyridine
B) RCOOH + R'OH + H+
C) RCOOH + R'OH + OH-
D) RCONH2 + R'OH

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
38
What is the missing reagent in the reaction below? 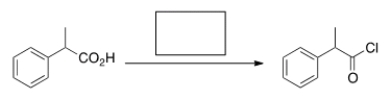
A) acetic acid
B) NaOMe
C) SOCl2
D) pyridine

A) acetic acid
B) NaOMe
C) SOCl2
D) pyridine

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
39
What is the product of the following reaction? 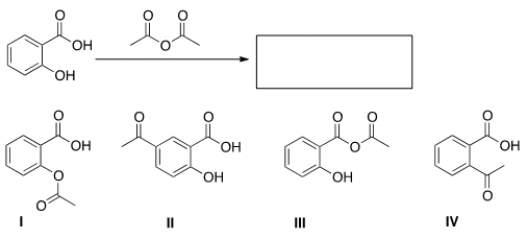
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
40
What is the IUPAC name for the following compound? 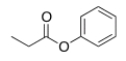
A) Propionyl phenol
B) Phenyl propanoate
C) Phenol propanoate
D) Phenyl acetate

A) Propionyl phenol
B) Phenyl propanoate
C) Phenol propanoate
D) Phenyl acetate

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following statements about amides is true?
A) Amides react with methyl alcohol in the presence of an acid catalyst to form an ester.
B) Amides are hydrolyzed in acid or base to form carboxylic acids or carboxylate anions.
C) Amides react with thionyl chloride to form the acid chloride.
D) Amides do not react under any conditions. They are inert compounds.
A) Amides react with methyl alcohol in the presence of an acid catalyst to form an ester.
B) Amides are hydrolyzed in acid or base to form carboxylic acids or carboxylate anions.
C) Amides react with thionyl chloride to form the acid chloride.
D) Amides do not react under any conditions. They are inert compounds.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following substrates cannot be used as an immediate precursor to synthesize an ester? 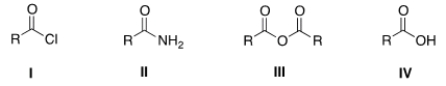
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
43
Draw the structure of N-phenyl acetamide. 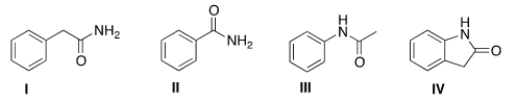
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
44
All of the following contain sp2 hybridized atoms in their functional group except
A) a carboxylic acid
B) a nitrile
C) an aldehyde
D) an anhydride
A) a carboxylic acid
B) a nitrile
C) an aldehyde
D) an anhydride

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
45
n-Butanenitrile will undergo all of the following listed reactions except one. Which reaction listed is not true for n-butanenitrile?
A) n-Butane nitrile will react with ethylmagnesium bromide to form an alcohol.
B) n-Butanenitrile with be reduced to butyl amine in the presence of LiAlH4 with an aqueous work-up.
C) n-Butanenitrile is hydrolyzed in the presence of acid to form butanoic acid.
D) n-Butanenitrile is reduced with DIBAL-H to form n-butanal.
A) n-Butane nitrile will react with ethylmagnesium bromide to form an alcohol.
B) n-Butanenitrile with be reduced to butyl amine in the presence of LiAlH4 with an aqueous work-up.
C) n-Butanenitrile is hydrolyzed in the presence of acid to form butanoic acid.
D) n-Butanenitrile is reduced with DIBAL-H to form n-butanal.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
46
An unknown compound has a sharp, medium peak at 2250 cm-1. The unknown compound is probably
A) an alkane
B) an aldehyde
C) an alkene
D) a nitrile
A) an alkane
B) an aldehyde
C) an alkene
D) a nitrile

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
47
From the list below, pick the compound that does not react with acetyl chloride.
A) Isopropyl alcohol
B) Benzoic acid
C) Ammonia
D) Triethyl amine
A) Isopropyl alcohol
B) Benzoic acid
C) Ammonia
D) Triethyl amine

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck



