Deck 28: Quantum Physics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/66
Play
Full screen (f)
Deck 28: Quantum Physics
1
A crystalographer uses X-rays that diffract from a crystal in which the spacing between atomic planes is 0.175 nm.If she observes a second-order diffraction at 22.0°,at what angle will she see the first-order diffraction?
A)62.4°
B)73.8°
C)84.7°
D)42.1°
A)62.4°
B)73.8°
C)84.7°
D)42.1°
62.4°
2
A photocathode whose work function is 2.9 eV is illuminated with white light that has a continuous wavelength band from 400 nm to 700 nm.The range of the wavelength band in this white light illumination for which photoelectrons are not produced,in nm,is closest to:
A)430 to 700
B)400 to 480
C)430 to 480
D)400 to 430
E)480 to 700
A)430 to 700
B)400 to 480
C)430 to 480
D)400 to 430
E)480 to 700
430 to 700
3
The work function of a particular substance is 4.20 10-19J.What is the photoelectric cutoff wavelength for this material?
A)473 nm
B)308 nm
C)393 nm
D)554 nm
A)473 nm
B)308 nm
C)393 nm
D)554 nm
473 nm
4
A light beam from a 2.1 mW He-Ne laser has wavelength of 633 nm.How many photons does the laser emit in one second?
A)6.7 × 1015
B)8.8 × 1015
C)1.1 × 1016
D)1.3 × 1016
A)6.7 × 1015
B)8.8 × 1015
C)1.1 × 1016
D)1.3 × 1016

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
5
A photocathode has a work function of 2.4 eV.The photocathode is illuminated with monochromatic radiation whose photon energy is 3.5 eV.The wavelength of the illuminating radiation is closest to:
A)350 nm
B)330 nm
C)300 nm
D)380 nm
E)410 nm
A)350 nm
B)330 nm
C)300 nm
D)380 nm
E)410 nm

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
6
Upon being struck by 240 nm photons,a material ejects electrons with a maximum kinetic energy of 2.58 eV.What is the work function of this material?
A)2.60 eV
B)2.18 eV
C)3.02 eV
D)3.43 eV
A)2.60 eV
B)2.18 eV
C)3.02 eV
D)3.43 eV

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
7
A helium-neon laser emits light at 632.8 nm.If the laser emits 1.82 1017 photons/second,what is its power output in mW?
A)57.2 mW
B)28.6 mW
C)37.2 mW
D)45.7 mW
A)57.2 mW
B)28.6 mW
C)37.2 mW
D)45.7 mW

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
8
You want to confine an electron in a box so that its minimum energy is 5.0 × 10-18 J.What is the length of the box?
A)0.11 nm
B)0.22 nm
C)0.15 nm
D)0.18 nm
A)0.11 nm
B)0.22 nm
C)0.15 nm
D)0.18 nm

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
9
A 84 kW AM radio station broadcasts at 1000 KHz.How many photons are emitted each second by the transmitting antenna?
A)1.3 × 1032
B)2.9 × 1024
C)6.3 × 1012
D)1.4 × 1015
A)1.3 × 1032
B)2.9 × 1024
C)6.3 × 1012
D)1.4 × 1015

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
10
A photocathode has a work function of 2.4 eV.The photocathode is illuminated with monochromatic radiation whose photon energy is 3.4 eV.The maximum kinetic energy of the photoelectrons produced is closest to:
A)1.6 × 10-19 J
B)3.8 × 10-19 J
C)4.4 × 10-19 J
D)4.9 × 10-19 J
E)5.4 × 10-19 J
A)1.6 × 10-19 J
B)3.8 × 10-19 J
C)4.4 × 10-19 J
D)4.9 × 10-19 J
E)5.4 × 10-19 J

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
11
Gamma rays are photons with very high energy.What is the wavelength of a gamma-ray photon with energy 7.7 × 10-13 J?
A)2.6 × 10-13 m
B)3.9 × 10-13 m
C)3.1 × 10-13 m
D)3.5 × 10-13 m
A)2.6 × 10-13 m
B)3.9 × 10-13 m
C)3.1 × 10-13 m
D)3.5 × 10-13 m

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
12
What is the velocity of an electron with a de Broglie wavelength of 4.32 μm?
A)168 m/s
B)123 m/s
C)145 m/s
D)211 m/s
A)168 m/s
B)123 m/s
C)145 m/s
D)211 m/s

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
13
Find the energy (in eV)of an optical photon of frequency 6.43 1014Hz.
A)2.66 eV
B)1.62 eV
C)1.94 eV
D)3.27 eV
A)2.66 eV
B)1.62 eV
C)1.94 eV
D)3.27 eV

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
14
A photocathode whose work function is 2.5 eV is illuminated with white light that has a continuous wavelength band from 360 nm to 700 nm.The stopping potential for this white light illumination is closest to:
A)0.94 V
B)0.85 V
C)1.0 V
D)1.1 V
E)1.2 V
A)0.94 V
B)0.85 V
C)1.0 V
D)1.1 V
E)1.2 V

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
15
Gamma rays are photons with very high energy.How many visible-light photons with a wavelength of 500 nm would you need to match the energy of a gamma-ray photon with energy 4.1 10-13 J?
A)1.0 × 106
B)1.4 × 108
C)6.2 × 109
D)3.9 × 103
A)1.0 × 106
B)1.4 × 108
C)6.2 × 109
D)3.9 × 103

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
16
Find the wavelength (in nm)of a 6.32 eV photon.
A)196 nm
B)167 nm
C)216 nm
D)234 nm
A)196 nm
B)167 nm
C)216 nm
D)234 nm

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
17
A photocathode has a work function of 2.8 eV.The photocathode is illuminated with monochromatic radiation whose photon energy is 4.0 eV.The threshold frequency for photoelectron production is closest to:
A)6.8 × 1014 Hz
B)2.9 × 1014 Hz
C)7.7 × 1014 Hz
D)8.6 × 1014 Hz
E)9.7 × 1014 Hz
A)6.8 × 1014 Hz
B)2.9 × 1014 Hz
C)7.7 × 1014 Hz
D)8.6 × 1014 Hz
E)9.7 × 1014 Hz

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
18
A crystal is irradiated with X-ray photons with energies of 1.65 × 10-15 J.The atomic planes in the crystal are separated by 0.21 nm.At what angles of incidence will the X-rays reflect from the crystal?
A)73°,55°,and 31°
B)55°
C)73° and 31°
D)73°
A)73°,55°,and 31°
B)55°
C)73° and 31°
D)73°

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
19
You want to confine an electron and you want to know for certain that the electron's speed is no more than 66 m/s.What is the length of the smallest box in which you can do this?
A)5.5 × 10-6 m
B)1.1 × 10-5 m
C)2.8 × 10-6 m
D)1.4 × 10-6 m
A)5.5 × 10-6 m
B)1.1 × 10-5 m
C)2.8 × 10-6 m
D)1.4 × 10-6 m

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
20
Find the de Broglie wavelength of a 1.30 kg object moving a 28.10 m/s.
A)1.81 × 10-35 m
B)2.05 × 10-35 m
C)2.29 × 10-35 m
D)2.58 × 10-35 m
A)1.81 × 10-35 m
B)2.05 × 10-35 m
C)2.29 × 10-35 m
D)2.58 × 10-35 m

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
21
Electrons with a speed of 2.1 × 106 m/s are directed towards a 1.0 μm wide slit.An electron detector is placed 1.0 m behind the slit.How wide is the electron diffraction pattern on the detector?
A)690 μm
B)350 μm
C)1000 μm
D)1400 μm
A)690 μm
B)350 μm
C)1000 μm
D)1400 μm

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
22
For what wavelength does a 100 mW laser deliver 1.6 × 1017 photons in one second?
A)320 nm
B)330 nm
C)340 nm
D)350 nm
A)320 nm
B)330 nm
C)340 nm
D)350 nm

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
23
You measure the velocity of an electron to an accuracy of ±22.6 m/s.What is the minimum uncertainty in its position?
A)8.05 μm
B)4.03 μm
C)16.1 μm
D)32.2 μm
A)8.05 μm
B)4.03 μm
C)16.1 μm
D)32.2 μm

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
24
A 10 g bouncy ball is confined in a 8.3 cm long box.What is its minimum energy?
A)8.0 × 10-64 J
B)3.2 × 10-46 J
C)1.3 × 10-20 J
D)zero
A)8.0 × 10-64 J
B)3.2 × 10-46 J
C)1.3 × 10-20 J
D)zero

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
25
An electron and a photon having the same wavelength will have the same energy.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
26
An electron has the same de Broglie wavelength as a 1.8 eV photon.The speed of the electron is closest to:
A)1100 m/s
B)980 m/s
C)910 m/s
D)840 m/s
E)770 m/s
A)1100 m/s
B)980 m/s
C)910 m/s
D)840 m/s
E)770 m/s

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
27
Electrons emerge from an electron gun with a speed of 2.0 × 106 m/s.They are made to pass through a double-slit apparatus.Interference fringes with spacing of 2.7 mm are detected on a screen far from the double slit.What would the fringe spacing be if the electrons were replaced by neutrons with the same speed?
A)1.5 μm
B)4.9 m
C)0.93 nm
D)1.2 km
A)1.5 μm
B)4.9 m
C)0.93 nm
D)1.2 km

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
28
An electron is confined in a one-dimensional box.Two adjacent allowed energies of the electron are 2.8 × 10-19 J and 3.5 × 10-19 J.What is the length of the box?
A)1.9 × 10-9 m
B)9.3 × 10-10 m
C)1.1 × 10-9 m
D)2.3 × 10-9 m
A)1.9 × 10-9 m
B)9.3 × 10-10 m
C)1.1 × 10-9 m
D)2.3 × 10-9 m

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
29
A molecule of roughly spherical shape has a mass of 1.80 × 10-25 kg and a diameter of 0.6 nm.The uncertainty in the measured position of the molecule is equal to the molecular diameter.The minimum speed of the molecule is closest to:
A)1 m/s
B)10 m/s
C)100 m/s
D)0.1 m/s
E)0.01 m/s
A)1 m/s
B)10 m/s
C)100 m/s
D)0.1 m/s
E)0.01 m/s

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
30
The frequency of light is the number of photons per second that it carries.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
31
A one-dimensional box contains an electron in the n = 1 state.What is the length of the box if the electron has the same energy as a photon with a wavelength of 600 nm?
A)430 pm
B)560 pm
C)0.85 nm
D)1.2 nm
A)430 pm
B)560 pm
C)0.85 nm
D)1.2 nm

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
32
A proton has a speed of 7.2 × 104 m/s.The energy of a photon that has the same de Broglie wavelength as the proton is closest to:
A)230 keV
B)150 keV
C)300 keV
D)370 keV
E)440 keV
A)230 keV
B)150 keV
C)300 keV
D)370 keV
E)440 keV

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
33
Electrons are accelerated to a speed of 4.0 × 104 m/s and are aimed at a double-slit apparatus.Interference fringes are detected.If the electrons were replaced by neutrons,what speed must neutrons have to produce interference fringes with the same fringe spacing as that observed with the electrons?
A)2.2 × 101 m/s
B)7.3 × 107 m/s
C)1.7 × 106 m/s
D)9.3 × 102 m/s
A)2.2 × 101 m/s
B)7.3 × 107 m/s
C)1.7 × 106 m/s
D)9.3 × 102 m/s

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
34
An electron is confined in a one-dimensional box.Two adjacent allowed energies of the electron are 2.8 × 10-19 J and 3.5 × 10-19 J.What is the length of the box?
A)1.9 × 10-9 m
B)9.3 × 10-10 m
C)1.1 × 10-9 m
D)2.3 × 10-9 m
A)1.9 × 10-9 m
B)9.3 × 10-10 m
C)1.1 × 10-9 m
D)2.3 × 10-9 m

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
35
Photons all travel at the speed of light so they must all have the same energy.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
36
An electron's de Broglie wavelength is 4.8 μm.What is its speed?
A)1.5 × 102 m/s
B)1.3 × 105 m/s
C)8.3 × 102 m/s
D)4.2 × 106 m/s
A)1.5 × 102 m/s
B)1.3 × 105 m/s
C)8.3 × 102 m/s
D)4.2 × 106 m/s

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
37
An electron has the same de Broglie wavelength as a 390 nm photon.The speed of the electron is closest to:
A)1900 m/s
B)2100 m/s
C)1700m/s
D)1500 m/s
E)540 m/s
A)1900 m/s
B)2100 m/s
C)1700m/s
D)1500 m/s
E)540 m/s

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
38
An electron is trapped in a quantum well that is 48.6 nm wide.Calculate the minimum uncertainty in its velocity.
A)7.49 × 103 m/s
B)1.79 × 104 m/s
C)2.98 × 104 m/s
D)3.34 × 104 m/s
A)7.49 × 103 m/s
B)1.79 × 104 m/s
C)2.98 × 104 m/s
D)3.34 × 104 m/s

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
39
Increasing the kinetic energy of an electron increases its de Broglie wavelength.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
40
An electron is accelerated from rest through a potential difference.After acceleration the electron has a wavelength of 880 nm.What is the potential difference responsible for the acceleration of the electron?
A)1.9 × 10-6 V
B)1.7 × 10-6 V
C)2.2 × 10-6 V
D)2.5 × 10-6 V
A)1.9 × 10-6 V
B)1.7 × 10-6 V
C)2.2 × 10-6 V
D)2.5 × 10-6 V

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
41
The maximum kinetic energy of photoelectrons from a metal is equal to the work function of that metal.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
42
An electron and a proton move at the same speed.Which has a longer de-Broglie wavelength?
A)the electron
B)the proton
C)Both particles have the same wavelength.
D)Since electrons and protons are particles,it is meaningless to discuss a wavelength associated with these particles.
A)the electron
B)the proton
C)Both particles have the same wavelength.
D)Since electrons and protons are particles,it is meaningless to discuss a wavelength associated with these particles.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
43
A photon of violet light has more energy than a photon of green light.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
44
A beam of light having frequency greater than the threshold frequency shines on a metal surface.By increasing the intensity of this light,you increase the number of photoelectrons emitted per second from this metal.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
45
Monochromatic light is incident on a metal surface.The ejected electrons give rise to a current in the circuit shown.The maximum energy of the ejected electrons is determined by applying a reverse ("stopping")potential,sufficient to reduce the current in the ammeter to zero. 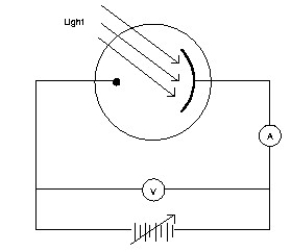 If the intensity of the incident light is increased,how will the required stopping potential change?
If the intensity of the incident light is increased,how will the required stopping potential change?
A)It will remain unchanged.
B)It will increase.
C)It will decrease.
 If the intensity of the incident light is increased,how will the required stopping potential change?
If the intensity of the incident light is increased,how will the required stopping potential change?A)It will remain unchanged.
B)It will increase.
C)It will decrease.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
46
A photon collides with an electron that is initially at rest,causing the electron to move.After the collision,the wavelength of the photon is
A)greater than its initial value.
B)equal to its initial value.
C)less than its initial value.
A)greater than its initial value.
B)equal to its initial value.
C)less than its initial value.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
47
A friend in your physics class tells you that illuminating a surface with any laser will result in the emission of photoelectrons,as long as the illumination lasts long enough to supply the needed energy.Is she correct? Support your answer.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
48
When light is absorbed by a metal,the energy of each light quantum is usually given to several adjacent electrons.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
49
A photon of blue light
A)has a smaller wavelength than a photon of red light and travels with the same speed.
B)has a smaller wavelength than a photon of red light and travels with a greater speed.
C)has a longer wavelength than a photon of red light and travels with the same speed.
D)has a longer wavelength than a photon of red light and travels with a greater speed.
A)has a smaller wavelength than a photon of red light and travels with the same speed.
B)has a smaller wavelength than a photon of red light and travels with a greater speed.
C)has a longer wavelength than a photon of red light and travels with the same speed.
D)has a longer wavelength than a photon of red light and travels with a greater speed.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
50
A beam of light having frequency greater than the threshold frequency shines on a metal surface.By increasing the frequency of this light,you increase the number of photoelectrons emitted per second from this metal.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
51
Illuminating a particular metal surface with a helium-neon laser results in the emission of photoelectrons.Will the kinetic energy of the photoelectrons increase if you increase the laser's power? Support your answer.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
52
To say that a particle's energy is "quantized" means that it can have only certain discrete values.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
53
A quantum of blue light carries more energy than a quantum of red light.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
54
If there are two photons with different energies,the one that has a higher energy
A)has the higher frequency.
B)has the higher wavelength.
C)has the higher velocity.
D)Two of the above are valid.
A)has the higher frequency.
B)has the higher wavelength.
C)has the higher velocity.
D)Two of the above are valid.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
55
If the threshold frequency of a certain metal is 2.0 × 1014 Hz,light of frequency 1.9 x 1014 Hz will not be able to dislodge any photoelectrons from this metal.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
56
Electrons are sent through a double-slit apparatus,and an interference pattern is observed.If protons are sent through the same apparatus with the same speed as that of the electrons,the distance between the fringes would be
A)closer together.
B)farther apart.
C)the same as that of the electrons since the velocities are the same.
A)closer together.
B)farther apart.
C)the same as that of the electrons since the velocities are the same.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
57
We do not observe the wave behavior of particles of matter in our daily lives because their de Broglie wavelengths are too small for their wave nature to show up.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
58
An electron is contrained to be inside a one-dimensional box.If the length of the box is doubled,the minimum energy of the electron
A)decreases by a factor of four.
B)decreases by a factor of two.
C)does not change.
D)is zero,regardless of the the size of the box.
A)decreases by a factor of four.
B)decreases by a factor of two.
C)does not change.
D)is zero,regardless of the the size of the box.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
59
Typical photoelectric work functions are in the range of a few joules.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
60
The threshold frequency of a metal is the largest frequency of light that will dislodge photoelectrons from this metal.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
61
A blue laser beam is incident on a metallic surface,causing electrons to be ejected from the metal.If the frequency of the laser beam is increased while the intensity of the beam is held fixed,
A)the rate of ejected electrons will decrease and the maximum kinetic energy will increase.
B)the rate of ejected electrons will remain the same but the maximum kinetic energy will increase.
C)the rate of ejected electrons will increase and the maximum kinetic energy will increase.
D)the rate of ejected electrons will remain the same but the maximum kinetic energy will decrease.
A)the rate of ejected electrons will decrease and the maximum kinetic energy will increase.
B)the rate of ejected electrons will remain the same but the maximum kinetic energy will increase.
C)the rate of ejected electrons will increase and the maximum kinetic energy will increase.
D)the rate of ejected electrons will remain the same but the maximum kinetic energy will decrease.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
62
Part of the energy level diagram of a certain atom is shown below.The energy spacing between levels 1 and 2 is twice that between 2 and 3.If an electron makes a transition from level 3 to level 2,the radiation of wavelength λ is emitted. 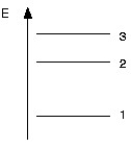 What possible radiation wavelengths might be produced by other transitions between the three energy levels?
What possible radiation wavelengths might be produced by other transitions between the three energy levels?
A)both λ/2 and λ/3
B)only λ/2
C)both 2λ and 3λ
D)only 2λ
 What possible radiation wavelengths might be produced by other transitions between the three energy levels?
What possible radiation wavelengths might be produced by other transitions between the three energy levels?A)both λ/2 and λ/3
B)only λ/2
C)both 2λ and 3λ
D)only 2λ

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
63
Increasing the brightness of a beam of light without changing its color will increase
A)the number of photons emitted by light every second.
B)the average energy of each photon.
C)the speed of the photons.
D)Two of the above statements are true.
A)the number of photons emitted by light every second.
B)the average energy of each photon.
C)the speed of the photons.
D)Two of the above statements are true.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
64
Monochromatic light is incident on a metal surface and electrons are ejected.If the intensity of the light is increased,what will happen to the ejection rate and maximum energy of the electrons?
A)greater rate;same maximum energy
B)same rate;greater maximum energy
C)greater rate;greater maximum energy
D)same rate;same maximum energy
A)greater rate;same maximum energy
B)same rate;greater maximum energy
C)greater rate;greater maximum energy
D)same rate;same maximum energy

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
65
When the surface of a metal is exposed to blue light,electrons are emitted.If the intensity of the blue light is increased,which of the following will also increase?
A)the number of electrons ejected per second
B)the maximum kinetic energy of the ejected electrons
C)the time lag between the onset of the absorption of light and the ejection of electrons
D)All of the above
E)Two of the above
A)the number of electrons ejected per second
B)the maximum kinetic energy of the ejected electrons
C)the time lag between the onset of the absorption of light and the ejection of electrons
D)All of the above
E)Two of the above

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
66
A proton and an electron are confined in a very small one-dimensional box.Which object has the lower minimum kinetic energy?
A)the proton
B)the electron
C)The minimum kinetic energy is the same for both particles.
A)the proton
B)the electron
C)The minimum kinetic energy is the same for both particles.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck



