Deck 4: Nomenclature and Conformations of Alkanes and Cycloalkanes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/163
Play
Full screen (f)
Deck 4: Nomenclature and Conformations of Alkanes and Cycloalkanes
1
The neopentyl group has the alternative name:
A)1,1-Dimethylpropyl
B)1,2-Dimethylpropyl
C)2,2-Dimethylpropyl
D)1-Methylbutyl
E)2-Methylbutyl
A)1,1-Dimethylpropyl
B)1,2-Dimethylpropyl
C)2,2-Dimethylpropyl
D)1-Methylbutyl
E)2-Methylbutyl
2,2-Dimethylpropyl
2
An IUPAC name for the group 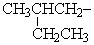 is:
is:
A)Isopentyl
B)Isoamyl
C)sec-Butylmethyl
D)2-Methylbutyl
E)2-Ethylpropyl
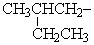 is:
is:A)Isopentyl
B)Isoamyl
C)sec-Butylmethyl
D)2-Methylbutyl
E)2-Ethylpropyl
2-Methylbutyl
3
What is the common name for this compound? 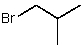
A)Isobutyl bromide
B)tert-Butyl bromide
C)Butyl bromide
D)sec-Butyl bromide
E)Bromo-sec-butane

A)Isobutyl bromide
B)tert-Butyl bromide
C)Butyl bromide
D)sec-Butyl bromide
E)Bromo-sec-butane
Isobutyl bromide
4
Provide a name for the following compound: 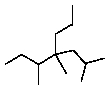
A)4-Isobutyl-3,4-dimethylheptane
B)4-sec-Butyl-2,4-dimethylheptane
C)2,4,5-Trimethyl-4-propylheptane
D)3,4,6-Trimethyl-4-propylheptane
E)4-Isobutyl-4,5-dimethylheptane

A)4-Isobutyl-3,4-dimethylheptane
B)4-sec-Butyl-2,4-dimethylheptane
C)2,4,5-Trimethyl-4-propylheptane
D)3,4,6-Trimethyl-4-propylheptane
E)4-Isobutyl-4,5-dimethylheptane

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
5
Neglecting stereochemistry,which of these common group names is ambiguous,i.e.,does not refer to one specific group?
A)Butyl
B)sec-Butyl
C)tert-Pentyl
D)Neopentyl
E)sec-Pentyl
A)Butyl
B)sec-Butyl
C)tert-Pentyl
D)Neopentyl
E)sec-Pentyl

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
6
A correct IUPAC name for the following compound is: 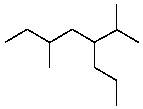
A)2,5-Dimethyl-3-propylheptane
B)3,6-Dimethyl-5-propylheptane
C)6-Methyl-4-(1-methylethyl)octane
D)2-Methyl-3-(2-methylbutyl)hexane
E)3-Methyl-5-(1-methylethyl)octane

A)2,5-Dimethyl-3-propylheptane
B)3,6-Dimethyl-5-propylheptane
C)6-Methyl-4-(1-methylethyl)octane
D)2-Methyl-3-(2-methylbutyl)hexane
E)3-Methyl-5-(1-methylethyl)octane

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
7
How many constitutional isomers are possible for the formula C6H14?
A)2
B)3
C)4
D)5
E)6
A)2
B)3
C)4
D)5
E)6

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
8
The IUPAC name for 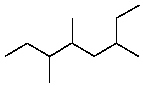 is:
is:
A)6-Ethyl-3,4-dimethylheptane
B)2-Ethyl-4,5-dimethylheptane
C)3,4,6-Trimethyloctane
D)3,5,6-Trimethyloctane
E)2-(1-Methylpropyl)-4-methylhexane
 is:
is:A)6-Ethyl-3,4-dimethylheptane
B)2-Ethyl-4,5-dimethylheptane
C)3,4,6-Trimethyloctane
D)3,5,6-Trimethyloctane
E)2-(1-Methylpropyl)-4-methylhexane

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
9
How many compounds with the formula C7H16 (heptanes)contain a single 3° carbon atom?
A)2
B)3
C)4
D)5
E)6
A)2
B)3
C)4
D)5
E)6

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
10
How many alkanes of formula C7H16 possess a quaternary carbon atom?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
11
How many compounds with the formula C4H11N contain a 2o amine and a single 2o carbon atom?
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
12
An IUPAC name for 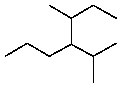 is:
is:
A)5-Methyl-4-(1-methylpropyl)hexane
B)2-Methyl-3-(1-methylpropyl)hexane
C)2-Methyl-3-(2-methylpropyl)hexane
D)3-Methyl-4-(1-methylethyl)heptane
E)5-Methyl-4-(1-methylethyl)heptane
 is:
is:A)5-Methyl-4-(1-methylpropyl)hexane
B)2-Methyl-3-(1-methylpropyl)hexane
C)2-Methyl-3-(2-methylpropyl)hexane
D)3-Methyl-4-(1-methylethyl)heptane
E)5-Methyl-4-(1-methylethyl)heptane

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following pairs of compounds represent pairs of constitutional isomers?
A)2-Methylbutane and pentane
B)2-Chlorohexane and 3-chlorohexane
C)sec-Butyl bromide and tert-butyl bromide
D)Propyl chloride and isopropyl chloride
E)All of these choices.
A)2-Methylbutane and pentane
B)2-Chlorohexane and 3-chlorohexane
C)sec-Butyl bromide and tert-butyl bromide
D)Propyl chloride and isopropyl chloride
E)All of these choices.

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
14
What is the correct IUPAC name for the following compound? 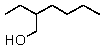
A)3-Hydroxymethylheptane
B)3-Hydroxymethylhexane
C)3-Methyloxyheptane
D)2-Ethyl-1-hexanol
E)2-Ethyl-1-heptanol

A)3-Hydroxymethylheptane
B)3-Hydroxymethylhexane
C)3-Methyloxyheptane
D)2-Ethyl-1-hexanol
E)2-Ethyl-1-heptanol

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
15
What is a correct name for the following compound? 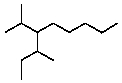
A)3-Isobutyl-2-methylheptane
B)3-sec-Butyl-2-methyloctane
C)5-Isobutyl-6-methylheptane
D)2-Ethyl-3-isopropyloctane
E)4-Isopropyl-3-methylnonane

A)3-Isobutyl-2-methylheptane
B)3-sec-Butyl-2-methyloctane
C)5-Isobutyl-6-methylheptane
D)2-Ethyl-3-isopropyloctane
E)4-Isopropyl-3-methylnonane

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
16
Isopentyl is the common name for which alkyl group?
A)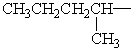
B)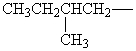
C)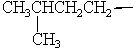
D)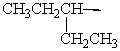
E)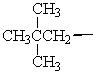
A)
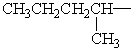
B)
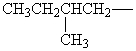
C)
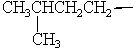
D)
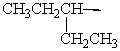
E)
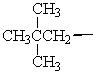

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
17
Provide a name for the following compound: 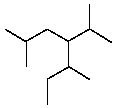
A)3-Isobutyl-2,4-dimethylhexane
B)3-sec-Butyl-2,5-dimethylhexane
C)4-sec-Butyl-2,5-dimethylhexane
D)4-Isopropyl-2,5-dimethylheptane
E)4-Isopropyl-3,6-dimethylheptane

A)3-Isobutyl-2,4-dimethylhexane
B)3-sec-Butyl-2,5-dimethylhexane
C)4-sec-Butyl-2,5-dimethylhexane
D)4-Isopropyl-2,5-dimethylheptane
E)4-Isopropyl-3,6-dimethylheptane

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
18
Which of these is the common name for the 1,1-dimethylpropyl group?
A)tert-Butyl
B)tert-Pentyl
C)Isopentyl
D)Neopentyl
E)sec-Pentyl
A)tert-Butyl
B)tert-Pentyl
C)Isopentyl
D)Neopentyl
E)sec-Pentyl

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
19
What is the simplest alkane,i.e.,the one with the smallest molecular weight,which possesses primary,secondary and tertiary carbon atoms?
A)2-Methylpropane
B)2-Methylbutane
C)2-Methylpentane
D)3-Methylpentane
E)2,2-Dimethylbutane
A)2-Methylpropane
B)2-Methylbutane
C)2-Methylpentane
D)3-Methylpentane
E)2,2-Dimethylbutane

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
20
Provide a name for the following compound: 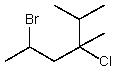
A)2-Bromo-4-chloro-4-isopropylpentane
B)4-Bromo-2-chloro-2-isopropylpentane
C)5-Bromo-3-chloro-2,3-dimethylhexane
D)2-Bromo-4-chloro-4,5-dimethylhexane
E)2-(2-Bromopropyl)-2-chloro-3-methylbutane

A)2-Bromo-4-chloro-4-isopropylpentane
B)4-Bromo-2-chloro-2-isopropylpentane
C)5-Bromo-3-chloro-2,3-dimethylhexane
D)2-Bromo-4-chloro-4,5-dimethylhexane
E)2-(2-Bromopropyl)-2-chloro-3-methylbutane

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
21
The correct IUPAC name for the following compound is: 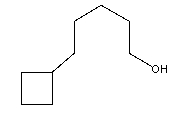
A)5-(1-pentanolyl)-cyclobutane
B)5-cyclobutyl-1-pentanol
C)1-cyclobutyl-5-pentanol
D)1-(5-pentanolyl)-cyclobutane
E)none of these choices

A)5-(1-pentanolyl)-cyclobutane
B)5-cyclobutyl-1-pentanol
C)1-cyclobutyl-5-pentanol
D)1-(5-pentanolyl)-cyclobutane
E)none of these choices

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following structures represents bicyclo[3.2.1]octane? ![<strong>Which of the following structures represents bicyclo[3.2.1]octane? </strong> A)I B)II C)III D)IV E)V](https://d2lvgg3v3hfg70.cloudfront.net/TB5902/11eaa4bc_3d95_dd65_9180_79438bb1c33e_TB5902_00.jpg)
A)I
B)II
C)III
D)IV
E)V
![<strong>Which of the following structures represents bicyclo[3.2.1]octane? </strong> A)I B)II C)III D)IV E)V](https://d2lvgg3v3hfg70.cloudfront.net/TB5902/11eaa4bc_3d95_dd65_9180_79438bb1c33e_TB5902_00.jpg)
A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following is bicyclo[4.3.0]nonane? ![<strong>Which of the following is bicyclo[4.3.0]nonane? </strong> A)I B)II C)III D)IV E)V](https://d2lvgg3v3hfg70.cloudfront.net/TB5902/11eaa4bc_3d95_dd64_9180_cb8ff6f39acb_TB5902_00.jpg)
A)I
B)II
C)III
D)IV
E)V
![<strong>Which of the following is bicyclo[4.3.0]nonane? </strong> A)I B)II C)III D)IV E)V](https://d2lvgg3v3hfg70.cloudfront.net/TB5902/11eaa4bc_3d95_dd64_9180_cb8ff6f39acb_TB5902_00.jpg)
A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
24
Which compound is a bicycloheptane? 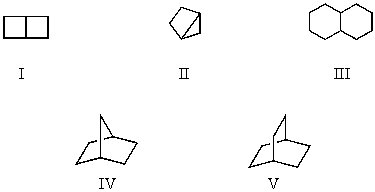
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following is bicyclo[3.2.2]nonane? ![<strong>Which of the following is bicyclo[3.2.2]nonane? </strong> A)I B)II C)III D)IV E)V](https://d2lvgg3v3hfg70.cloudfront.net/TB5902/11eaa4bc_3d95_8f3e_9180_b7d0acab1bfe_TB5902_00.jpg)
A)I
B)II
C)III
D)IV
E)V
![<strong>Which of the following is bicyclo[3.2.2]nonane? </strong> A)I B)II C)III D)IV E)V](https://d2lvgg3v3hfg70.cloudfront.net/TB5902/11eaa4bc_3d95_8f3e_9180_b7d0acab1bfe_TB5902_00.jpg)
A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
26
What is the correct name of the following compound? ![<strong>What is the correct name of the following compound? </strong> A)1-Chlorobicyclo[4.1.1]octane B)2-Chlorobicyclo[4.1.0]octane C)2-Chlorobicyclo[4.1.1]octane D)2-Chlorobicyclo[4.1.1]heptane E)5-Chlorobicyclo[4.1.1]octane](https://d2lvgg3v3hfg70.cloudfront.net/TB5902/11eaa4bc_3d96_0477_9180_f7ca71ab269d_TB5902_00.jpg)
A)1-Chlorobicyclo[4.1.1]octane
B)2-Chlorobicyclo[4.1.0]octane
C)2-Chlorobicyclo[4.1.1]octane
D)2-Chlorobicyclo[4.1.1]heptane
E)5-Chlorobicyclo[4.1.1]octane
![<strong>What is the correct name of the following compound? </strong> A)1-Chlorobicyclo[4.1.1]octane B)2-Chlorobicyclo[4.1.0]octane C)2-Chlorobicyclo[4.1.1]octane D)2-Chlorobicyclo[4.1.1]heptane E)5-Chlorobicyclo[4.1.1]octane](https://d2lvgg3v3hfg70.cloudfront.net/TB5902/11eaa4bc_3d96_0477_9180_f7ca71ab269d_TB5902_00.jpg)
A)1-Chlorobicyclo[4.1.1]octane
B)2-Chlorobicyclo[4.1.0]octane
C)2-Chlorobicyclo[4.1.1]octane
D)2-Chlorobicyclo[4.1.1]heptane
E)5-Chlorobicyclo[4.1.1]octane

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
27
What is the correct IUPAC name for the following compound? 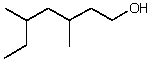
A)5-Ethyl-3-methylhexanol
B)5-Ethyl-3-methyl-1-hexanol
C)2-Ethyl-4-methyl-6-hexanol
D)3,5-Dimethyl-7-heptanol
E)3,5-Dimethyl-1-heptanol

A)5-Ethyl-3-methylhexanol
B)5-Ethyl-3-methyl-1-hexanol
C)2-Ethyl-4-methyl-6-hexanol
D)3,5-Dimethyl-7-heptanol
E)3,5-Dimethyl-1-heptanol

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
28
Provide a name for the following compound: 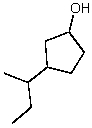
A)1-Hydroxy-3-sec-butylcyclopentane
B)3-sec-Butyl-1-cyclopentanol
C)1-sec-Butyl-3-cyclopentanol
D)4-sec-Butyl-1-cyclopentanol
E)3-Isobutyl-1-cyclopentanol

A)1-Hydroxy-3-sec-butylcyclopentane
B)3-sec-Butyl-1-cyclopentanol
C)1-sec-Butyl-3-cyclopentanol
D)4-sec-Butyl-1-cyclopentanol
E)3-Isobutyl-1-cyclopentanol

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
29
A correct name for the following compound is: ![<strong>A correct name for the following compound is: </strong> A)7,7-dichlorobicyclo[2.2.1]heptane B)2,2-dichlorobicyclo[1.2.2]heptane C)1,1-dichlorobicyclo[2.2.1]heptane D)1,1-dichlorobicyclo[1.2.2]heptane E)None of these choices](https://d2lvgg3v3hfg70.cloudfront.net/TB5902/11eaa4bc_3d96_2b8a_9180_3f6d1306ae04_TB5902_00.jpg)
A)7,7-dichlorobicyclo[2.2.1]heptane
B)2,2-dichlorobicyclo[1.2.2]heptane
C)1,1-dichlorobicyclo[2.2.1]heptane
D)1,1-dichlorobicyclo[1.2.2]heptane
E)None of these choices
![<strong>A correct name for the following compound is: </strong> A)7,7-dichlorobicyclo[2.2.1]heptane B)2,2-dichlorobicyclo[1.2.2]heptane C)1,1-dichlorobicyclo[2.2.1]heptane D)1,1-dichlorobicyclo[1.2.2]heptane E)None of these choices](https://d2lvgg3v3hfg70.cloudfront.net/TB5902/11eaa4bc_3d96_2b8a_9180_3f6d1306ae04_TB5902_00.jpg)
A)7,7-dichlorobicyclo[2.2.1]heptane
B)2,2-dichlorobicyclo[1.2.2]heptane
C)1,1-dichlorobicyclo[2.2.1]heptane
D)1,1-dichlorobicyclo[1.2.2]heptane
E)None of these choices

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
30
Which is the correct name for the compound shown below? ![<strong>Which is the correct name for the compound shown below? </strong> A)Bicyclo[2.2.0]hexane B)Bicyclo[2.2.0]butane C)Bicyclo[2.2.2]hexane D)Bicyclo[2.2.1]hexane E)Disquarane](https://d2lvgg3v3hfg70.cloudfront.net/TB5902/11eaa4bc_3d95_8f40_9180_e9afbc0198e9_TB5902_00.jpg)
A)Bicyclo[2.2.0]hexane
B)Bicyclo[2.2.0]butane
C)Bicyclo[2.2.2]hexane
D)Bicyclo[2.2.1]hexane
E)Disquarane
![<strong>Which is the correct name for the compound shown below? </strong> A)Bicyclo[2.2.0]hexane B)Bicyclo[2.2.0]butane C)Bicyclo[2.2.2]hexane D)Bicyclo[2.2.1]hexane E)Disquarane](https://d2lvgg3v3hfg70.cloudfront.net/TB5902/11eaa4bc_3d95_8f40_9180_e9afbc0198e9_TB5902_00.jpg)
A)Bicyclo[2.2.0]hexane
B)Bicyclo[2.2.0]butane
C)Bicyclo[2.2.2]hexane
D)Bicyclo[2.2.1]hexane
E)Disquarane

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
31
What is the correct IUPAC name for the following compound? 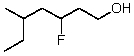
A)5-Ethyl-3-fluorohexanol
B)5-Ethyl-3-fluoro-1-hexanol
C)2-Ethyl-4-fluoro-6-hexanol
D)3-Fluoro-5-methyl-7-heptanol
E)3-Fluoro-5-methyl-1-heptanol

A)5-Ethyl-3-fluorohexanol
B)5-Ethyl-3-fluoro-1-hexanol
C)2-Ethyl-4-fluoro-6-hexanol
D)3-Fluoro-5-methyl-7-heptanol
E)3-Fluoro-5-methyl-1-heptanol

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following is bicyclo[5.2.0]nonane? ![<strong>Which of the following is bicyclo[5.2.0]nonane? </strong> A)I B)II C)III D)IV E)V](https://d2lvgg3v3hfg70.cloudfront.net/TB5902/11eaa4bc_3d95_b653_9180_6b102c9f322e_TB5902_00.jpg)
A)I
B)II
C)III
D)IV
E)V
![<strong>Which of the following is bicyclo[5.2.0]nonane? </strong> A)I B)II C)III D)IV E)V](https://d2lvgg3v3hfg70.cloudfront.net/TB5902/11eaa4bc_3d95_b653_9180_6b102c9f322e_TB5902_00.jpg)
A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following is a correct name which corresponds to the common name tert-pentyl alcohol?
A)2,2-Dimethyl-1-propanol
B)2-Ethyl-2-propanol
C)2-Methyl-2-butanol
D)3-Methyl-1-butanol
E)Methyl tert-butanol
A)2,2-Dimethyl-1-propanol
B)2-Ethyl-2-propanol
C)2-Methyl-2-butanol
D)3-Methyl-1-butanol
E)Methyl tert-butanol

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
34
The correct IUPAC name for the following compound is: 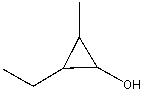
A)1-methyl-2-ethyl-3-cyclopropanol
B)2-methyl-3-ethyl-1-cyclopropanol
C)3-ethyl-2-methyl-1-cyclopropanol
D)1-ethyl-2-methyl-3-cyclopropanol
E)none of these choices

A)1-methyl-2-ethyl-3-cyclopropanol
B)2-methyl-3-ethyl-1-cyclopropanol
C)3-ethyl-2-methyl-1-cyclopropanol
D)1-ethyl-2-methyl-3-cyclopropanol
E)none of these choices

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
35
A correct name for the following compound is: ![<strong>A correct name for the following compound is: </strong> A)4-bromo-3,8-dimethylbicyclo[5.2.2]nonane B)3,8-dimethyl-4-bromo-bicyclo[5.2.0]nonane C)4-bromo-3,8-dimethylbicyclo[5.2.1]decane D)7-bromo-2,6-dimethylbicyclo[5.2.0]nonane E)4-bromo-3,8-dimethylbicyclo[5.2.0]nonane](https://d2lvgg3v3hfg70.cloudfront.net/TB5902/11eaa4bc_3d96_0479_9180_a5f62115dc55_TB5902_00.jpg)
A)4-bromo-3,8-dimethylbicyclo[5.2.2]nonane
B)3,8-dimethyl-4-bromo-bicyclo[5.2.0]nonane
C)4-bromo-3,8-dimethylbicyclo[5.2.1]decane
D)7-bromo-2,6-dimethylbicyclo[5.2.0]nonane
E)4-bromo-3,8-dimethylbicyclo[5.2.0]nonane
![<strong>A correct name for the following compound is: </strong> A)4-bromo-3,8-dimethylbicyclo[5.2.2]nonane B)3,8-dimethyl-4-bromo-bicyclo[5.2.0]nonane C)4-bromo-3,8-dimethylbicyclo[5.2.1]decane D)7-bromo-2,6-dimethylbicyclo[5.2.0]nonane E)4-bromo-3,8-dimethylbicyclo[5.2.0]nonane](https://d2lvgg3v3hfg70.cloudfront.net/TB5902/11eaa4bc_3d96_0479_9180_a5f62115dc55_TB5902_00.jpg)
A)4-bromo-3,8-dimethylbicyclo[5.2.2]nonane
B)3,8-dimethyl-4-bromo-bicyclo[5.2.0]nonane
C)4-bromo-3,8-dimethylbicyclo[5.2.1]decane
D)7-bromo-2,6-dimethylbicyclo[5.2.0]nonane
E)4-bromo-3,8-dimethylbicyclo[5.2.0]nonane

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
36
A correct name for the following compound is: ![<strong>A correct name for the following compound is: </strong> A)2-Methylbicyclo[4.3.0]nonane B)1-Methylbicyclo[4.3.1]nonane C)7-Methylbicyclo[4.3.0]nonane D)2-Methylbicyclo[4.3.1]nonane E)1-Methylbicyclo[4.3.0]nonane](https://d2lvgg3v3hfg70.cloudfront.net/TB5902/11eaa4bc_3d95_dd66_9180_a7ab8a072d75_TB5902_00.jpg)
A)2-Methylbicyclo[4.3.0]nonane
B)1-Methylbicyclo[4.3.1]nonane
C)7-Methylbicyclo[4.3.0]nonane
D)2-Methylbicyclo[4.3.1]nonane
E)1-Methylbicyclo[4.3.0]nonane
![<strong>A correct name for the following compound is: </strong> A)2-Methylbicyclo[4.3.0]nonane B)1-Methylbicyclo[4.3.1]nonane C)7-Methylbicyclo[4.3.0]nonane D)2-Methylbicyclo[4.3.1]nonane E)1-Methylbicyclo[4.3.0]nonane](https://d2lvgg3v3hfg70.cloudfront.net/TB5902/11eaa4bc_3d95_dd66_9180_a7ab8a072d75_TB5902_00.jpg)
A)2-Methylbicyclo[4.3.0]nonane
B)1-Methylbicyclo[4.3.1]nonane
C)7-Methylbicyclo[4.3.0]nonane
D)2-Methylbicyclo[4.3.1]nonane
E)1-Methylbicyclo[4.3.0]nonane

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
37
A correct IUPAC name for the following compound is: 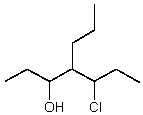
A)4-propyl-5-chloro-3-heptanol
B)4-propyl-3-chloro-5-heptanol
C)4-(1-chloropropyl)-3-heptanol
D)5-chloro-4-propyl-3-heptanol
E)3-hydroxy-4-propyl-5-chloroheptane

A)4-propyl-5-chloro-3-heptanol
B)4-propyl-3-chloro-5-heptanol
C)4-(1-chloropropyl)-3-heptanol
D)5-chloro-4-propyl-3-heptanol
E)3-hydroxy-4-propyl-5-chloroheptane

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
38
What is the name of this compound? ![<strong>What is the name of this compound? </strong> A)Bicyclo[2.2.2]octane B)Bicyclo[3.2.1]octane C)Bicyclo[4.1.1]octane D)Bicyclo[4.2.0]octane E)Bicyclo[3.3.0]octane](https://d2lvgg3v3hfg70.cloudfront.net/TB5902/11eaa4bc_3d95_b651_9180_77cd8e9d3240_TB5902_00.jpg)
A)Bicyclo[2.2.2]octane
B)Bicyclo[3.2.1]octane
C)Bicyclo[4.1.1]octane
D)Bicyclo[4.2.0]octane
E)Bicyclo[3.3.0]octane
![<strong>What is the name of this compound? </strong> A)Bicyclo[2.2.2]octane B)Bicyclo[3.2.1]octane C)Bicyclo[4.1.1]octane D)Bicyclo[4.2.0]octane E)Bicyclo[3.3.0]octane](https://d2lvgg3v3hfg70.cloudfront.net/TB5902/11eaa4bc_3d95_b651_9180_77cd8e9d3240_TB5902_00.jpg)
A)Bicyclo[2.2.2]octane
B)Bicyclo[3.2.1]octane
C)Bicyclo[4.1.1]octane
D)Bicyclo[4.2.0]octane
E)Bicyclo[3.3.0]octane

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
39
A correct name for the following compound is: ![<strong>A correct name for the following compound is: </strong> A)3-Chloro-8-methylbicyclo[4.3.0]nonane B)8-Methyl-3-chlorobicyclo[4.3.1]nonane C)3-Methyl-7-chlorobicyclo[4.3.0]nonane D)3-Methyl-7-chlorobicyclo[4.3.1]decane E)3-Chloro-8-methyl[4.3.0]bicyclononane](https://d2lvgg3v3hfg70.cloudfront.net/TB5902/11eaa4bc_3d96_0478_9180_b7ea7b8211fc_TB5902_00.jpg)
A)3-Chloro-8-methylbicyclo[4.3.0]nonane
B)8-Methyl-3-chlorobicyclo[4.3.1]nonane
C)3-Methyl-7-chlorobicyclo[4.3.0]nonane
D)3-Methyl-7-chlorobicyclo[4.3.1]decane
E)3-Chloro-8-methyl[4.3.0]bicyclononane
![<strong>A correct name for the following compound is: </strong> A)3-Chloro-8-methylbicyclo[4.3.0]nonane B)8-Methyl-3-chlorobicyclo[4.3.1]nonane C)3-Methyl-7-chlorobicyclo[4.3.0]nonane D)3-Methyl-7-chlorobicyclo[4.3.1]decane E)3-Chloro-8-methyl[4.3.0]bicyclononane](https://d2lvgg3v3hfg70.cloudfront.net/TB5902/11eaa4bc_3d96_0478_9180_b7ea7b8211fc_TB5902_00.jpg)
A)3-Chloro-8-methylbicyclo[4.3.0]nonane
B)8-Methyl-3-chlorobicyclo[4.3.1]nonane
C)3-Methyl-7-chlorobicyclo[4.3.0]nonane
D)3-Methyl-7-chlorobicyclo[4.3.1]decane
E)3-Chloro-8-methyl[4.3.0]bicyclononane

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following is bicyclo[3.3.1]nonane? ![<strong>Which of the following is bicyclo[3.3.1]nonane? </strong> A)I B)II C)III D)IV E)V](https://d2lvgg3v3hfg70.cloudfront.net/TB5902/11eaa4bc_3d95_b652_9180_a5a36139795f_TB5902_00.jpg)
A)I
B)II
C)III
D)IV
E)V
![<strong>Which of the following is bicyclo[3.3.1]nonane? </strong> A)I B)II C)III D)IV E)V](https://d2lvgg3v3hfg70.cloudfront.net/TB5902/11eaa4bc_3d95_b652_9180_a5a36139795f_TB5902_00.jpg)
A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
41
The least stable conformation of 3-bromo-2-methylpentane,viewed through the C-3-C-4 bond (i.e.,C-3 in the front,C-4 in the back): 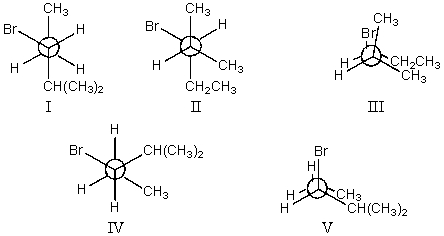
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
42
Give the IUPAC name for 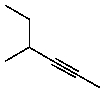
A)3-Methyl-4-hexyne
B)4-Methyl-2-hexyne
C)2-Ethyl-3-pentyne
D)4-Ethyl-2-pentyne
E)3-Methyl-2-hexyne

A)3-Methyl-4-hexyne
B)4-Methyl-2-hexyne
C)2-Ethyl-3-pentyne
D)4-Ethyl-2-pentyne
E)3-Methyl-2-hexyne

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
43
The most stable conformation of 1,2-dibromoethane is: 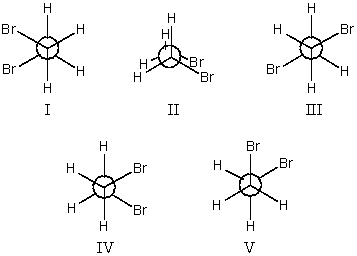
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
44
The least stable conformation of butane is: 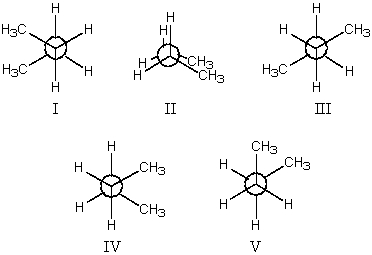
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
45
Which staggered Newman projection(s),looking down the C-2-C-3 bond (C-2 in front and C-3 in back),illustrates the following boxed compound? 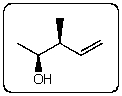
A)I,II and V
B)II and V
C)III and IV
D)V only
E)None of these choices.


A)I,II and V
B)II and V
C)III and IV
D)V only
E)None of these choices.

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
46
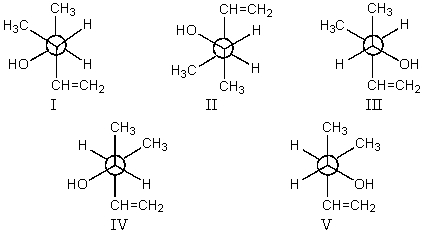
Which staggered Newman projection(s),looking down the C-2-C-3 bond (C-2 in front and C-3 in back),illustrate(s)the following boxed compound? 
A)I only
B)II and V
C)I and II
D)III only
E)None of these choices.


A)I only
B)II and V
C)I and II
D)III only
E)None of these choices.

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
47
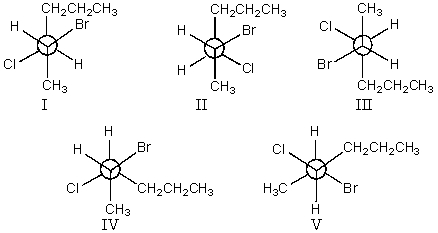
Which staggered Newman projection(s),looking down the C-3-C-4 bond (C-3 in front and C-4 in back),illustrate(s)the following boxed compound? 
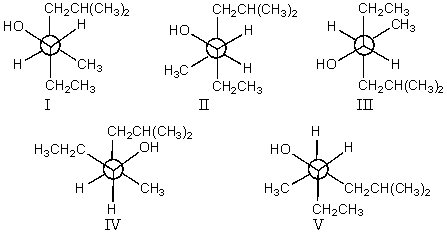
A)I,II and III
B)II and V
C)I and II
D)III only
E)None of these choices.


A)I,II and III
B)II and V
C)I and II
D)III only
E)None of these choices.

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
48
Consider the graph below,which is a plot of the relative energies of the various conformations of 2,3-dimethylbutane,viewed through the C-2-C-3 bond.The conformations corresponding to the 120o and 240o are: 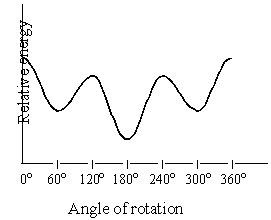
A)eclipsed,more stable than the conformation at 0o
B)eclipsed,more stable than the conformation at 180o
C)staggered,more stable than the conformation at 0o
D)staggered,less stable than the conformation at 180o
E)two of these choices are true

A)eclipsed,more stable than the conformation at 0o
B)eclipsed,more stable than the conformation at 180o
C)staggered,more stable than the conformation at 0o
D)staggered,less stable than the conformation at 180o
E)two of these choices are true

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
49
The most stable conformation of 2,3-dibromobutane,viewed through the C-2-C-3 bond : 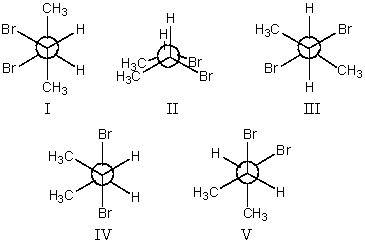
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
50
Which conformation(s)of 1,2-dibromoethane does not illustrate one or more gauche interactions? 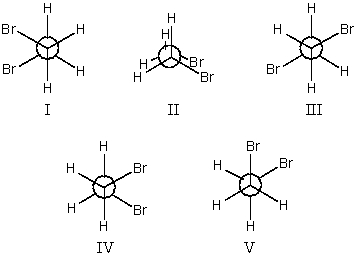
A)I and II only
B)II,III,and V only
C)I,III,and IV only
D)II and III only
E)All these choices

A)I and II only
B)II,III,and V only
C)I,III,and IV only
D)II and III only
E)All these choices

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
51
A correct IUPAC name for the following compound is: 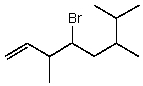
A)3,6,7-trimethyl-4-bromo-1-octene
B)4-bromo-3-methyl-6-isopropyl-1-heptene
C)4-bromo-3,6,7-trimethyl-1-octene
D)4-bromo-6-isopropyl-3-methyl-1-heptene
E)4-bromo-6-isopropyl-3,6-dimethyl-1-hexene

A)3,6,7-trimethyl-4-bromo-1-octene
B)4-bromo-3-methyl-6-isopropyl-1-heptene
C)4-bromo-3,6,7-trimethyl-1-octene
D)4-bromo-6-isopropyl-3-methyl-1-heptene
E)4-bromo-6-isopropyl-3,6-dimethyl-1-hexene

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
52
Consider the graph below,which is a plot of the relative energies of the various conformations of hexane,viewed through the C-2-C-3 bond.The conformations corresponding to the 60o and 300o are: 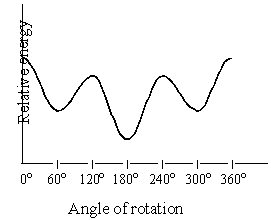
A)eclipsed
B)staggered and gauche
C)staggered and anti
D)more stable than the conformation at 180o
E)none of these choices

A)eclipsed
B)staggered and gauche
C)staggered and anti
D)more stable than the conformation at 180o
E)none of these choices

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
53
The most stable conformation of butane is: 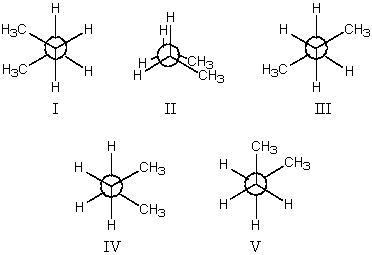
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
54
Which staggered Newman projection(s),looking down the C-2-C-3 bond (C-2 in front and C-3 in back),illustrates the following boxed compound? 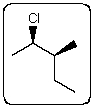
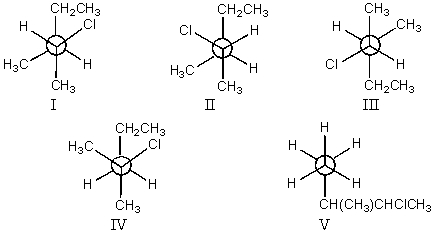
A)I,II and III
B)II and V
C)I and II
D)V only
E)None of these choices.


A)I,II and III
B)II and V
C)I and II
D)V only
E)None of these choices.

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
55
What is the correct IUPAC name for the following compound? 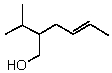
A)trans-3-Hydroxymethyl-2-heptene
B)trans-2-(1-methylethyl)-4-hexen-1-ol
C)trans-5-(1-methylethyl)-2-hexen-6-ol
D)cis-5-isopropyl-2,6-hexenol
E)cis-2-(1-methylethyl)-4-hepten-1-ol

A)trans-3-Hydroxymethyl-2-heptene
B)trans-2-(1-methylethyl)-4-hexen-1-ol
C)trans-5-(1-methylethyl)-2-hexen-6-ol
D)cis-5-isopropyl-2,6-hexenol
E)cis-2-(1-methylethyl)-4-hepten-1-ol

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
56
The IUPAC name for diisobutylacetylene is
A)2,7-Dimethyl-4-octene
B)2,7-Dimethyl-4-octyne
C)3,6-Dimethyl-4-octyne
D)2,5-Diethyl-3-hexyne
E)2,2,5,5-Tetramethyl-3-hexyne
A)2,7-Dimethyl-4-octene
B)2,7-Dimethyl-4-octyne
C)3,6-Dimethyl-4-octyne
D)2,5-Diethyl-3-hexyne
E)2,2,5,5-Tetramethyl-3-hexyne

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
57
The graph below is a plot of the relative energies of the various conformations of: 
A)Ethane
B)Propane
C)Chloroethane
D)1-Chloropropane (C1-C2 rotation)
E)Butane (C1-C2 rotation)

A)Ethane
B)Propane
C)Chloroethane
D)1-Chloropropane (C1-C2 rotation)
E)Butane (C1-C2 rotation)

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
58
Which conformation(s)show(s)the bromines in a gauche orientation for 2,3-dibromobutane,viewed through the C-2-C-3 bond? 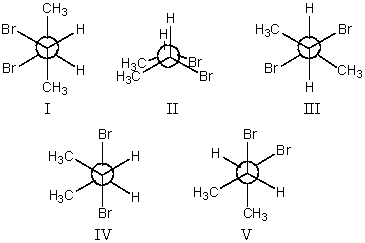
A)I and II only
B)III and IV only
C)I and V only
D)II only
E)None of these choices.

A)I and II only
B)III and IV only
C)I and V only
D)II only
E)None of these choices.

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
59
The most stable conformation of 3-bromo-2-methylpentane,viewed through the C-3-C-4 bond (i.e.,C-3 in the front,C-4 in the back): 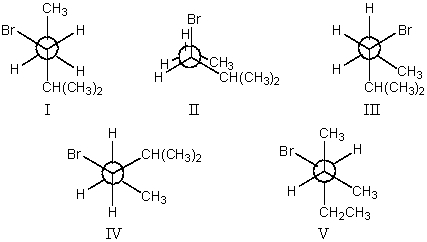
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
60
Give the IUPAC name for 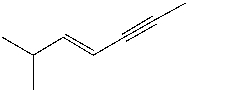
A)trans-2-methyl-3-hepten-5-yne
B)trans-6-methyl-4-hepten-2-yne
C)cis-2-methyl-3-hepten-5-yne
D)cis-6-methyl-4-hepten-2-yne
E)none of these choices

A)trans-2-methyl-3-hepten-5-yne
B)trans-6-methyl-4-hepten-2-yne
C)cis-2-methyl-3-hepten-5-yne
D)cis-6-methyl-4-hepten-2-yne
E)none of these choices

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
61
The most stable conformation for 1,2-ethanediol (ethylene glycol)is shown below.It is the most stable conformation because 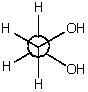
A)this corresponds to an anti conformation.
B)in general,gauche conformations possess the minimum energy.
C)it is stabilized by intramolecular hydrogen bonding.
D)it is a staggered conformation.
E)it has the highest energy of all the possibilities.

A)this corresponds to an anti conformation.
B)in general,gauche conformations possess the minimum energy.
C)it is stabilized by intramolecular hydrogen bonding.
D)it is a staggered conformation.
E)it has the highest energy of all the possibilities.

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
62
cis-1,2-Dibromocyclohexane is represented by structure(s): 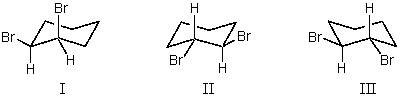
A)I
B)II
C)III
D)II and III
E)I and II

A)I
B)II
C)III
D)II and III
E)I and II

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
63
The least stable conformation of 2,3-dimethylpentane,viewed through the C-2-C-3 bond (i.e.,C-2 in the front,C-3 in the back): 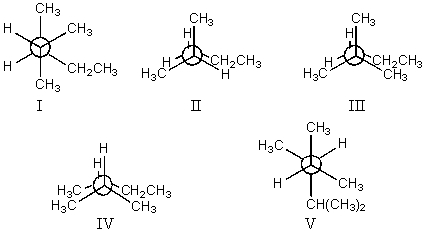
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
64
Select the systematic name for 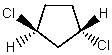
A)cis-1,3-Dichlorocyclopentane
B)trans-1,4-Dichlorocyclopentane
C)cis-1,2-Dichlorocyclopentane
D)trans-1,3-Dichlorocyclopentane
E)1,1-Dichlorocyclopentane

A)cis-1,3-Dichlorocyclopentane
B)trans-1,4-Dichlorocyclopentane
C)cis-1,2-Dichlorocyclopentane
D)trans-1,3-Dichlorocyclopentane
E)1,1-Dichlorocyclopentane

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
65
Which cycloalkane has the least ring strain?
A)Cyclopropane
B)Cyclobutane
C)Cyclopentane
D)Cyclohexane
E)Cycloheptane
A)Cyclopropane
B)Cyclobutane
C)Cyclopentane
D)Cyclohexane
E)Cycloheptane

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
66
What structure represents the most stable conformation of cis-1,3-dimethylcyclohexane? 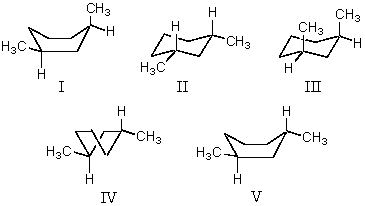
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
67
trans-1,2-Dibromocyclohexane is represented by structure(s): 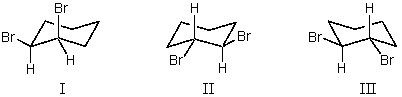
A)I
B)II
C)III
D)II and III
E)I and II

A)I
B)II
C)III
D)II and III
E)I and II

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
68
The preferred conformation of cis-3-tert-butyl-1-methylcyclohexane is the one in which:
A)the tert-butyl group is axial and the methyl group is equatorial.
B)the methyl group is axial and the tert-butyl group is equatorial.
C)both groups are axial.
D)both groups are equatorial.
E)the molecule exists in a boat conformation.
A)the tert-butyl group is axial and the methyl group is equatorial.
B)the methyl group is axial and the tert-butyl group is equatorial.
C)both groups are axial.
D)both groups are equatorial.
E)the molecule exists in a boat conformation.

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
69
Which is the most stable conformation of cyclohexane?
A)Chair
B)Twist
C)Boat
D)Half-chair
E)Staggered
A)Chair
B)Twist
C)Boat
D)Half-chair
E)Staggered

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
70
The most stable conformation of 3-bromo-2-methylpentane,viewed through the C-2-C-3 bond (i.e.,C-2 in the front,C-3 in the back): 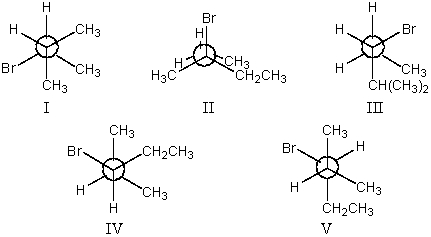
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
71
The graph below is a plot of the relative energies of the various conformations of: 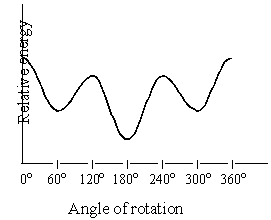
A)2-chloropropane
B)1,3-dichloropropane
C)2-methylpropane
D)Butane (C1-C2 rotation)
E)Butane (C2-C3 rotation)

A)2-chloropropane
B)1,3-dichloropropane
C)2-methylpropane
D)Butane (C1-C2 rotation)
E)Butane (C2-C3 rotation)

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
72
The twist boat conformation is the preferred conformation for this compound.
A)cis-1,4-Di-tert-butylcyclohexane
B)trans-1,4-Di-tert-butylcyclohexane
C)cis-1,3-Di-tert-butylcyclohexane
D)trans-1,2-Di-tert-butylcyclohexane
E)None of these choices.
A)cis-1,4-Di-tert-butylcyclohexane
B)trans-1,4-Di-tert-butylcyclohexane
C)cis-1,3-Di-tert-butylcyclohexane
D)trans-1,2-Di-tert-butylcyclohexane
E)None of these choices.

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
73
What is the most thermodynamically stable chair conformation for the substituents on cis-4-tert-butylcyclohexanol?
A)both groups equatorial
B)both groups axial
C)tert-butyl group equatorial and OH group axial
D)tert-butyl group axial and OH group equatorial
E)a boat conformation is adopted due to steric strain
A)both groups equatorial
B)both groups axial
C)tert-butyl group equatorial and OH group axial
D)tert-butyl group axial and OH group equatorial
E)a boat conformation is adopted due to steric strain

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
74
Which cycloalkane has the greatest ring strain?
A)Cyclopropane
B)Cyclobutane
C)Cyclopentane
D)Cyclohexane
E)Cycloheptane
A)Cyclopropane
B)Cyclobutane
C)Cyclopentane
D)Cyclohexane
E)Cycloheptane

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
75
 Which of the following is a conformer of trans-1,2-diethylcyclohexane?
Which of the following is a conformer of trans-1,2-diethylcyclohexane? 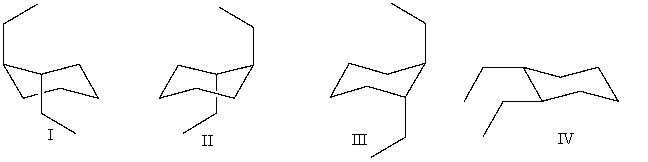
A)I and II
B)I,II and III
C)I and IV
D)all of these choices
E)none of these choices

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
76
The least stable conformation of 3-bromo-2-methylpentane,viewed through the C-2-C-3 bond (i.e.,C-2 in the front,C-3 in the back): 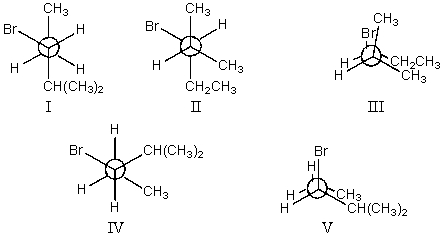
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
77
The least stable conformation of cyclohexane is the
A)boat.
B)twist boat.
C)chair.
D)half-chair.
E)twist chair.
A)boat.
B)twist boat.
C)chair.
D)half-chair.
E)twist chair.

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
78
What is the name of the IUPAC name of the following compound? 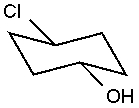
A)trans-4-chlorocyclohexan-1-ol
B)cis-1-chlorocyclohexan-4-ol
C)trans-4-chlorocyclohexanol
D)cis-4-chlorocyclohexanol
E)none of these choices

A)trans-4-chlorocyclohexan-1-ol
B)cis-1-chlorocyclohexan-4-ol
C)trans-4-chlorocyclohexanol
D)cis-4-chlorocyclohexanol
E)none of these choices

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
79
The most stable conformation of 2,3-dimethylpentane,viewed through the C-2-C-3 bond (i.e.,C-2 in the front,C-3 in the back): 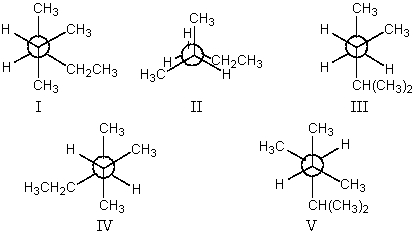
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck
80
Select the structure for cis-3-methyl-6-vinylcyclohexene. 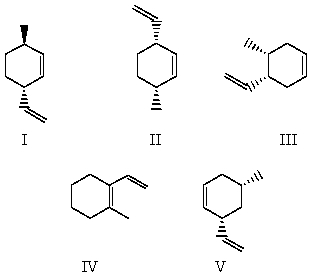
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 163 flashcards in this deck.
Unlock Deck
k this deck



