Deck 7: Alkenes and Alkynes I
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/220
Play
Full screen (f)
Deck 7: Alkenes and Alkynes I
1
Name the following compound: 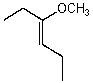
A)(cis)-3-methoxyhex-3-ene
B)(Z)-4-methoxyhex-4-ene
C)(Z)-3-methoxyhex-3-ene
D)(E)-3-methoxyhex-3-ene
E)3-methoxyhex-3-ene

A)(cis)-3-methoxyhex-3-ene
B)(Z)-4-methoxyhex-4-ene
C)(Z)-3-methoxyhex-3-ene
D)(E)-3-methoxyhex-3-ene
E)3-methoxyhex-3-ene
(Z)-3-methoxyhex-3-ene
2
Name the following compound: 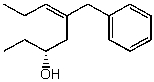
A)(R,Z)-5-phenyloct-5-en-3-ol
B)(R,Z)-5-benzyloct-5-en-3-ol
C)(R,E)-5-phenyloct-5-en-3-ol
D)(R,E)-5-benzyloct-5-en-3-ol
E)(R,E)-5-benzyloct-3-en-6-ol

A)(R,Z)-5-phenyloct-5-en-3-ol
B)(R,Z)-5-benzyloct-5-en-3-ol
C)(R,E)-5-phenyloct-5-en-3-ol
D)(R,E)-5-benzyloct-5-en-3-ol
E)(R,E)-5-benzyloct-3-en-6-ol
(R,Z)-5-benzyloct-5-en-3-ol
3
Name the following compound: 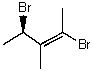
A)(S,E)-2,4-dibromo-3-methylpent-2-ene
B)(R,Z)-2,4-dibromo-3-methylpent-2-ene
C)(R,E)-2,4-dibromo-3-methylpent-3-ene
D)(S,E)-2,4-dibromo-3-methylpent-3-ene
E)(R,E)-2,4-dibromo-3-methylpent-2-ene

A)(S,E)-2,4-dibromo-3-methylpent-2-ene
B)(R,Z)-2,4-dibromo-3-methylpent-2-ene
C)(R,E)-2,4-dibromo-3-methylpent-3-ene
D)(S,E)-2,4-dibromo-3-methylpent-3-ene
E)(R,E)-2,4-dibromo-3-methylpent-2-ene
(R,E)-2,4-dibromo-3-methylpent-2-ene
4
Which alkene would liberate the most heat per mole when subjected to catalytic hydrogenation?
A)
B)
C)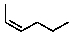
D)
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
5
Which structure represents (R,Z)-5-methylhept-2-ene?
A)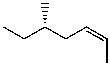
B)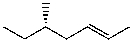
C)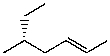
D)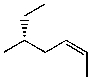
E)None of these choices.
A)

B)

C)

D)

E)None of these choices.

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
6
A correct IUPAC name for the following compound is: 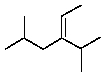
A)3,3,5-trimethyl-2-hexene
B)3-isobutyl-3-isopropyl-2-propene
C)3-isobutyl-4-methyl-2-pentene
D)3-(1-methylethyl)-5-methyl-2-hexene
E)None of these choices.

A)3,3,5-trimethyl-2-hexene
B)3-isobutyl-3-isopropyl-2-propene
C)3-isobutyl-4-methyl-2-pentene
D)3-(1-methylethyl)-5-methyl-2-hexene
E)None of these choices.

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
7
Heats of hydrogenation data would be useful in comparing the relative stabilities of which of the following substances? 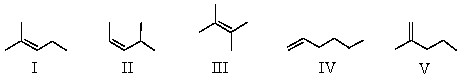
A)I,II,III
B)III,IV
C)I,II,V
D)Heats of hydrogenation data would not be a useful way to compare relative stabilities of any of the substances.
E)All of these substances could effectively be compared using heats of hydrogenation data.

A)I,II,III
B)III,IV
C)I,II,V
D)Heats of hydrogenation data would not be a useful way to compare relative stabilities of any of the substances.
E)All of these substances could effectively be compared using heats of hydrogenation data.

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
8
The correct IUPAC name for the following compound is: 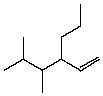
A)4,5-Dimethyl-3-propyl-2-hexene
B)4,5-Dimethyl-3-propyl-1-hexene
C)3-(2,3-Dimethylpropyl)-1-hexene
D)2,3-Dimethyl-4-isopropyl-5-hexene
E)2,3-Dimethyl-4-propyl-5-hexene

A)4,5-Dimethyl-3-propyl-2-hexene
B)4,5-Dimethyl-3-propyl-1-hexene
C)3-(2,3-Dimethylpropyl)-1-hexene
D)2,3-Dimethyl-4-isopropyl-5-hexene
E)2,3-Dimethyl-4-propyl-5-hexene

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
9
Name the following compound: 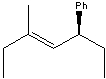
A)(S,E)-3-methyl-5-phenyl-3-heptene
B)(R,E)-3-methyl-5-phenyl-3-heptene
C)(S,E)-3-methyl-5-benzyl-3-heptene
D)(S,Z)-3-methyl-5-phenyl-3-heptene
E)(R,Z)-3-methyl-5-phenyl-3-heptene

A)(S,E)-3-methyl-5-phenyl-3-heptene
B)(R,E)-3-methyl-5-phenyl-3-heptene
C)(S,E)-3-methyl-5-benzyl-3-heptene
D)(S,Z)-3-methyl-5-phenyl-3-heptene
E)(R,Z)-3-methyl-5-phenyl-3-heptene

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
10
Which molecule would have the lowest heat of hydrogenation? 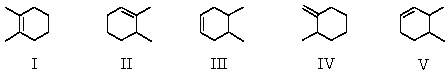
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
11
The correct IUPAC name for the following compound is: 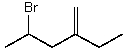
A)2-Bromo-4-methylenehexane
B)2-(2-Bromopropyl)-1-butene
C)4-Bromo-2-ethyl-1-pentene
D)2-Bromo-4-ethyl-1-pentene
E)2-Bromo-4-ethyl-4-pentene

A)2-Bromo-4-methylenehexane
B)2-(2-Bromopropyl)-1-butene
C)4-Bromo-2-ethyl-1-pentene
D)2-Bromo-4-ethyl-1-pentene
E)2-Bromo-4-ethyl-4-pentene

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
12
Which structure represents (Z)-1,4-dichlorohex-3-en-1-yne?
A)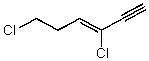
B)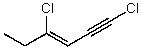
C)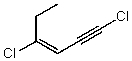
D)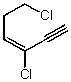
E)None of these choices.
A)

B)

C)

D)

E)None of these choices.

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
13
Which structure represents (E)-1-bromo-2-methylhex-2-ene?
A)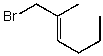
B)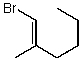
C)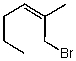
D)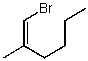
E)None of these choices.
A)

B)

C)

D)

E)None of these choices.

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
14
Name the following compound: 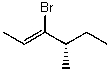
A)(S,Z)-3-bromo-4-methylhex-2-ene
B)(S)-3-bromo-4-methylhex-2-ene
C)(S,Z)-4-bromo-3-methylhex-4-ene
D)(S,E)-3-bromo-4-methylhex-2-ene
E)(R,E)-3-bromo-4-methylhex-2-ene

A)(S,Z)-3-bromo-4-methylhex-2-ene
B)(S)-3-bromo-4-methylhex-2-ene
C)(S,Z)-4-bromo-3-methylhex-4-ene
D)(S,E)-3-bromo-4-methylhex-2-ene
E)(R,E)-3-bromo-4-methylhex-2-ene

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
15
The correct IUPAC name for the following compound is: 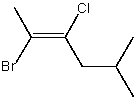
A)(E)-2-Bromo-3-chloro-5-methyl-2-hexene
B)(E)-2-Bromo-3-chloro-5-methyl-3-hexene
C)(Z)-2-Bromo-3-chloro-5-methyl-3-hexene
D)(Z)-2-Bromo-3-chloro-5-methyl-2-hexene
E)(E)-2-Methyl-5-bromo-4-chloro-4-hexene

A)(E)-2-Bromo-3-chloro-5-methyl-2-hexene
B)(E)-2-Bromo-3-chloro-5-methyl-3-hexene
C)(Z)-2-Bromo-3-chloro-5-methyl-3-hexene
D)(Z)-2-Bromo-3-chloro-5-methyl-2-hexene
E)(E)-2-Methyl-5-bromo-4-chloro-4-hexene

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
16
Name the following compound: 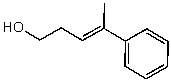
A)(E)-4-phenyl-4-methylbut-3-en-1-ol
B)(E)-4-phenylpent-3-en-1-ol
C)(Z)-4-phenylpent-3-en-1-ol
D)(Z)-4-phenyl-4-methylbut-3-en-1-ol
E)(E)-4-benzylpent-3-en-1-ol

A)(E)-4-phenyl-4-methylbut-3-en-1-ol
B)(E)-4-phenylpent-3-en-1-ol
C)(Z)-4-phenylpent-3-en-1-ol
D)(Z)-4-phenyl-4-methylbut-3-en-1-ol
E)(E)-4-benzylpent-3-en-1-ol

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
17
Name the following compound: 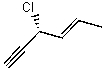
A)(S,E)-3-chlorohex-4-en-1-yne
B)(R,E)-3-chlorohex-2-en-5-yne
C)(S,E)-3-chlorohex-2-en-5-yne
D)(R,E)-3-chlorohex-4-en-1-yne
E)(R,E)-3-chloropent-4-en-1-yne

A)(S,E)-3-chlorohex-4-en-1-yne
B)(R,E)-3-chlorohex-2-en-5-yne
C)(S,E)-3-chlorohex-2-en-5-yne
D)(R,E)-3-chlorohex-4-en-1-yne
E)(R,E)-3-chloropent-4-en-1-yne

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
18
Name the following compound: 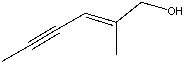
A)(Z)-5-methylhex-4-en-2-yn-6-ol
B)(E)-5-methylhex-4-en-2-yn-6-ol
C)(E)-2-methylhex-2-en-4-yn-1-ol
D)(Z)-2-methylhex-2-en-4-yn-1-ol
E)(Z)-1-methylhex-2-en-3-yn-1-ol

A)(Z)-5-methylhex-4-en-2-yn-6-ol
B)(E)-5-methylhex-4-en-2-yn-6-ol
C)(E)-2-methylhex-2-en-4-yn-1-ol
D)(Z)-2-methylhex-2-en-4-yn-1-ol
E)(Z)-1-methylhex-2-en-3-yn-1-ol

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
19
Which structure represents (Z)-4-bromohexa-1,3-diene?
A)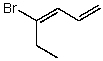
B)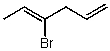
C)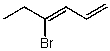
D)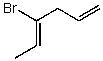
E)None of these choices.
A)

B)

C)

D)

E)None of these choices.

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
20
Select the structure of 4-ethyl-2,3-dimethyl-2-heptene.
A)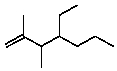
B)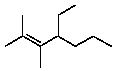
C)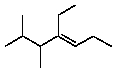
D)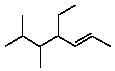
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
21
What is the major product for the following reaction? 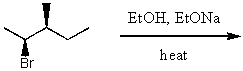
A)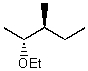
B)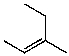
C)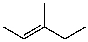
D)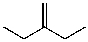
E)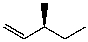
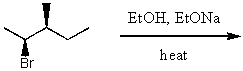
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
22
Dehydrohalogenation of tert-pentyl bromide at higher temperatures will produce 2-methyl-1-butene as the chief product when:
A)CH3COONa is employed as the base.
B)KOH/C2H5OH is employed as the base
C)CH3CH2ONa/CH3CH2OH is employed as the base.
D)(CH3)3COK/(CH3)3COH is employed as the base.
E)any base is used,as long as the temperature is sufficiently high.
A)CH3COONa is employed as the base.
B)KOH/C2H5OH is employed as the base
C)CH3CH2ONa/CH3CH2OH is employed as the base.
D)(CH3)3COK/(CH3)3COH is employed as the base.
E)any base is used,as long as the temperature is sufficiently high.

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
23
What is the major product for the following reaction? 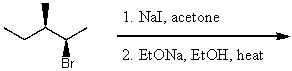
A)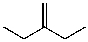
B)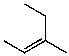
C)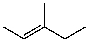
D)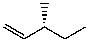
E)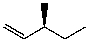
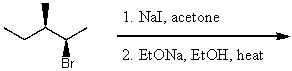
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
24
What is the major product for the following reaction? 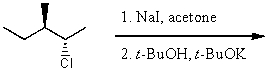
A)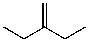
B)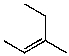
C)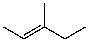
D)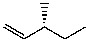
E)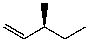
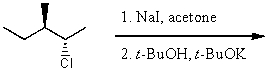
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
25
What is the major product for the following reaction? 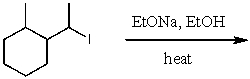
A)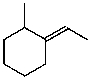
B)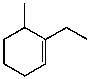
C)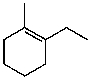
D)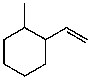
E)None of these choices.
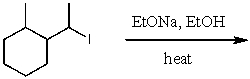
A)

B)

C)

D)

E)None of these choices.

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
26
Concerning the relative stabilities of alkenes,which is a false statement?
A)Unless hydrogenation of the alkenes gives the same alkane,heats of hydrogenation cannot be used to measure their relative stabilities.
B)In general,the greater the number of alkyl groups attached to the carbon atoms of the double bond,the greater the stability of the alkene.
C)The greater the quantity of heat liberated on combustion or hydrogenation of an alkene,the greater its energy content.
D)trans-Cycloalkenes are always more stable than the cis-isomers.
E)Heats of combustion can be used to measure the relative stabilities of isomeric alkenes,even though their hydrogenation products are not identical.
A)Unless hydrogenation of the alkenes gives the same alkane,heats of hydrogenation cannot be used to measure their relative stabilities.
B)In general,the greater the number of alkyl groups attached to the carbon atoms of the double bond,the greater the stability of the alkene.
C)The greater the quantity of heat liberated on combustion or hydrogenation of an alkene,the greater its energy content.
D)trans-Cycloalkenes are always more stable than the cis-isomers.
E)Heats of combustion can be used to measure the relative stabilities of isomeric alkenes,even though their hydrogenation products are not identical.

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
27
Which compound listed below would you expect to be the major product when 2-bromo-2-methylbutane is refluxed with KOH/ethanol?
A)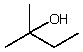
B)
C)
D)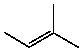
E)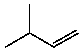
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
28
What is the major product for the following reaction? 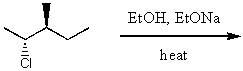
A)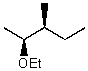
B)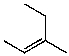
C)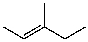
D)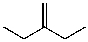
E)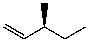
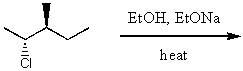
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
29
What is the major product for the following reaction? 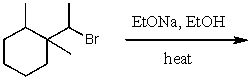
A)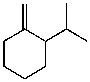
B)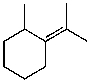
C)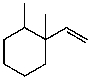
D)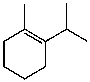
E)More than one of these choices.
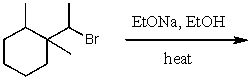
A)

B)

C)

D)

E)More than one of these choices.

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
30
What is the major product for the following reaction? 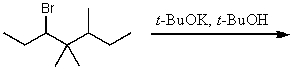
A)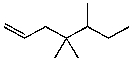
B)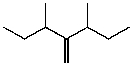
C)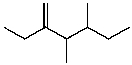
D)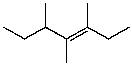
E)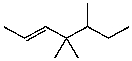
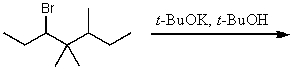
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
31
What is the major product for the following reaction? 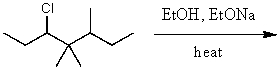
A)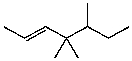
B)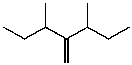
C)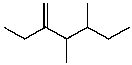
D)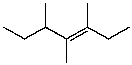
E)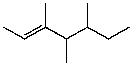
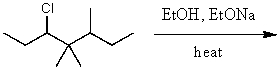
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
32
Zaitsev's rule states that:
A)In electrophilic addition of an unsymmetrical reagent to an unsymmetrical alkene,the more positive portion of the reagent will become attached to the carbon of the double bond bearing the greater number of hydrogen atoms.
B)An equatorial substituent in cyclohexane results in a more stable conformation than if that substituent were axial.
C)E2 reactions occur only if the -hydrogen and leaving group can assume an anti-periplanar arrangement.
D)When a reaction forms an alkene,and several possibilities exist,the more (or most)stable isomer is the one which predominates.
E)The order of reactivity of alcohols in dehydration reactions is 3º > 2º > 1º.
A)In electrophilic addition of an unsymmetrical reagent to an unsymmetrical alkene,the more positive portion of the reagent will become attached to the carbon of the double bond bearing the greater number of hydrogen atoms.
B)An equatorial substituent in cyclohexane results in a more stable conformation than if that substituent were axial.
C)E2 reactions occur only if the -hydrogen and leaving group can assume an anti-periplanar arrangement.
D)When a reaction forms an alkene,and several possibilities exist,the more (or most)stable isomer is the one which predominates.
E)The order of reactivity of alcohols in dehydration reactions is 3º > 2º > 1º.

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
33
What is the major product for the following reaction? 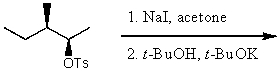
A)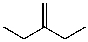
B)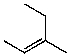
C)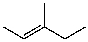
D)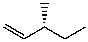
E)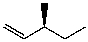
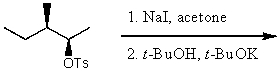
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
34
What is the major product for the following reaction? 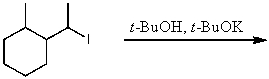
A)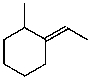
B)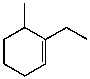
C)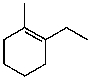
D)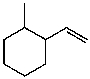
E)None of these choices.
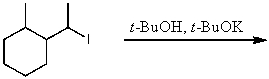
A)

B)

C)

D)

E)None of these choices.

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
35
Your task is to convert 2-bromobutane to 1-butene in highest yield.Which reagents would you use?
A)KOH/H2O
B)KOH/CH3OH
C)CH3ONa/CH3OH
D)CH3CH2ONa/CH3CH2OH
E)(CH3)3COK/(CH3)3COH
A)KOH/H2O
B)KOH/CH3OH
C)CH3ONa/CH3OH
D)CH3CH2ONa/CH3CH2OH
E)(CH3)3COK/(CH3)3COH

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
36
Which product (or products)would be formed in appreciable amount(s)when trans-1-bromo-2-methylcyclohexane undergoes dehydrohalogenation upon treatment with sodium ethoxide in ethanol? 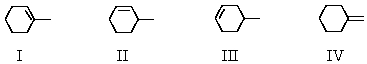
A)I
B)II
C)III
D)IV
E)More than one of these choices.
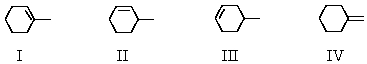
A)I
B)II
C)III
D)IV
E)More than one of these choices.

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
37
What is the major product for the following reaction? 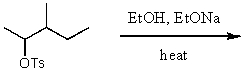
A)CH3CH2(CH3)C=CHCH3
B)CH3CH2(CH3)CHCH=CH2
C)CH3CH2(CH3)CHCH(OCH2CH3)CH3
D)None of these choices.
E)No reaction.
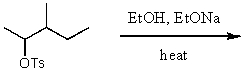
A)CH3CH2(CH3)C=CHCH3
B)CH3CH2(CH3)CHCH=CH2
C)CH3CH2(CH3)CHCH(OCH2CH3)CH3
D)None of these choices.
E)No reaction.

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
38
What is the major product for the following reaction? 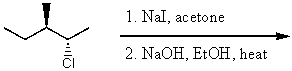
A)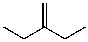
B)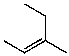
C)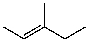
D)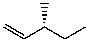
E)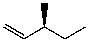
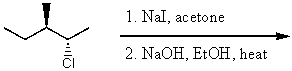
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
39
What is the major product for the following reaction? 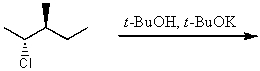
A)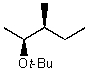
B)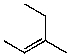
C)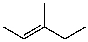
D)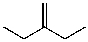
E)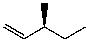
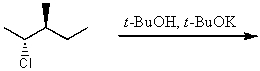
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
40
Rank the following compounds from lowest to highest heat of hydrogenation: 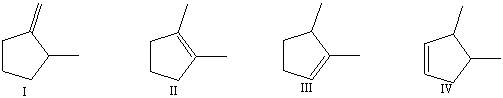
A)I,II,III,IV
B)IV,III,II,I
C)II,III,IV,I
D)I,IV,III,II
E)none of these choices

A)I,II,III,IV
B)IV,III,II,I
C)II,III,IV,I
D)I,IV,III,II
E)none of these choices

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
41
Regarding the use of potassium tert-butoxide as a base in E2 reactions,it is incorrect to state that:
A)this base is more effective than ethoxide ion,because it (KOt-Bu)is the more basic of the two.
B)it tends to give the anti-Zaitsev,i.e.,Hofmann,product.
C)it is more reactive in dimethyl sulfoxide than it is in tert-butyl alcohol.
D)it favors E2 reactions over competing SN2 reactions.
E)it will form,predominantly,the more stable alkene.
A)this base is more effective than ethoxide ion,because it (KOt-Bu)is the more basic of the two.
B)it tends to give the anti-Zaitsev,i.e.,Hofmann,product.
C)it is more reactive in dimethyl sulfoxide than it is in tert-butyl alcohol.
D)it favors E2 reactions over competing SN2 reactions.
E)it will form,predominantly,the more stable alkene.

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
42
Which one of the following alcohols would dehydrate most rapidly when treated with sulfuric acid? 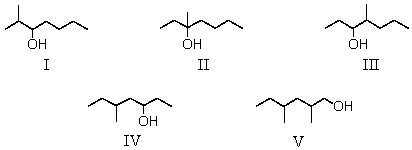
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
43
What is the major product of the reaction of the following reaction? 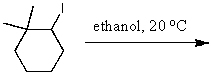
A)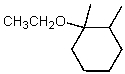
B)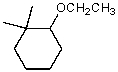
C)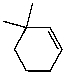
D)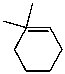
E)More than one of these choices.

A)

B)

C)

D)

E)More than one of these choices.

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
44
What is the major product of the reaction between methanol and (2R,3S)-2-bromo-3-methylpentane at room temperature?
A)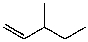
B)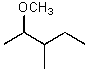
C)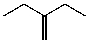
D)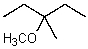
E)Two of these choices.
A)

B)

C)

D)

E)Two of these choices.

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
45
Which mechanistic step in the acid-catalyzed dehydration of 3,3-dimethyl-2-butanol is the rate determining step?
A)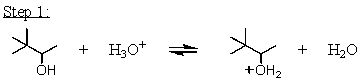
B)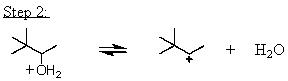
C)
D)
E)
A)
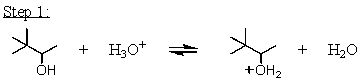
B)

C)

D)

E)


Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
46
Rearrangements are likely to occur in which of the following reaction types?
A)SN1 reactions
B)SN2 reactions
C)E1 reactions
D)E2 reactions
E)Both SN1 and E1 reactions
A)SN1 reactions
B)SN2 reactions
C)E1 reactions
D)E2 reactions
E)Both SN1 and E1 reactions

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
47
Which alcohol would be most easily dehydrated? 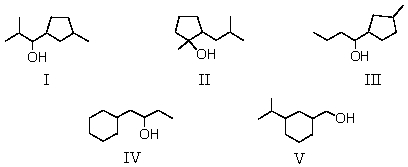
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
48
Neopentyl alcohol,(CH3)3CCH2OH,cannot be dehydrated to an alkene without rearrangement.What is the chief product of dehydration? 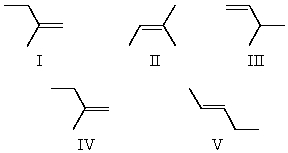
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
49
Which alcohol would initially produce the most stable carbocation when treated with concentrated H2SO4?
A)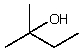
B)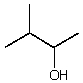
C)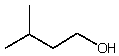
D)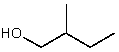
E)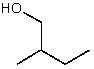
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
50
What are the major product(s)formed when trans-1-bromo-2-methylcyclohexane reacts with sodium isopropoxide in isopropanol? 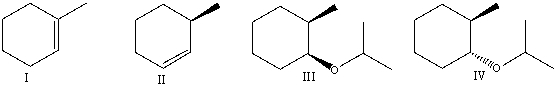
A)I
B)II
C)I and II
D)III
E)III and IV

A)I
B)II
C)I and II
D)III
E)III and IV

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
51
What is the major product of the reaction, 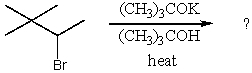
A)(CH3)2C=C(CH3)2
B)(CH3)3C-CH=CH2
C)(CH3)2C=CHCH3
D)(CH3)2C=CHCH2CH3
E)None of these choices.
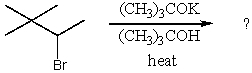
A)(CH3)2C=C(CH3)2
B)(CH3)3C-CH=CH2
C)(CH3)2C=CHCH3
D)(CH3)2C=CHCH2CH3
E)None of these choices.

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
52
Carbocations are frequent intermediates in acidic reactions of alkenes,alcohols,etc.What do carbocations usually do? They may:
A)rearrange to a more stable carbocation.
B)lose a proton to form an alkene.
C)combine with a nucleophile.
D)react with an alkene to form a larger carbocation.
E)do all of these choices.
A)rearrange to a more stable carbocation.
B)lose a proton to form an alkene.
C)combine with a nucleophile.
D)react with an alkene to form a larger carbocation.
E)do all of these choices.

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following carbocations would NOT be likely to undergo rearrangement?
A)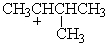
B)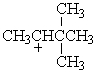
C)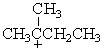
D)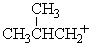
E)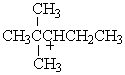
A)
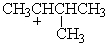
B)
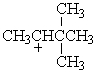
C)
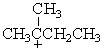
D)
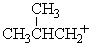
E)
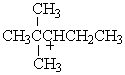

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
54
Which statement(s)is/are true of acid-catalyzed alcohol dehydrations?
A)Protonation of the alcohol is a fast step.
B)Formation of a carbocation from the protonated alcohol is a slow step.
C)Rearrangements of less stable carbocations to more stable carbocations are common.
D)Loss of a proton by the carbocation intermediate is a fast step.
E)All of these choices.
A)Protonation of the alcohol is a fast step.
B)Formation of a carbocation from the protonated alcohol is a slow step.
C)Rearrangements of less stable carbocations to more stable carbocations are common.
D)Loss of a proton by the carbocation intermediate is a fast step.
E)All of these choices.

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
55
Which product(s)would be produced by acid-catalyzed dehydration of 2-methyl-2-pentanol?
A)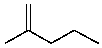
B)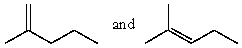
C)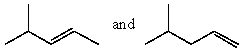
D)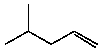
E)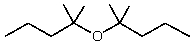
A)

B)
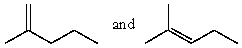
C)
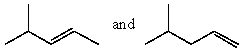
D)

E)


Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
56
What are the major product(s)formed when cis-1-bromo-2-methylcyclohexane reacts with sodium isopropoxide in isopropanol? 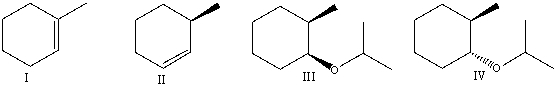
A)I
B)II
C)I and II
D)III
E)III and IV

A)I
B)II
C)I and II
D)III
E)III and IV

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
57
Which alcohol would be most easily dehydrated?
A)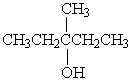
B)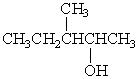
C)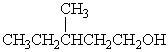
D)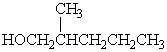
E)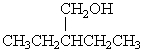
A)

B)

C)
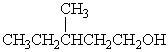
D)
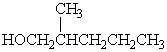
E)
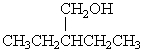

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following alcohols would probably undergo dehydration using 25% sulfuric acid below 100 oC? 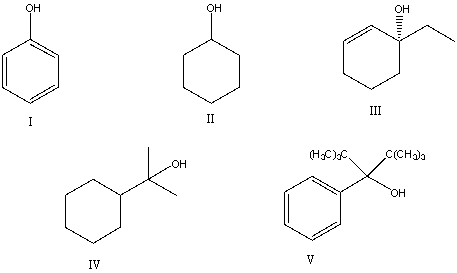
A)II,IV,V
B)I,II,III
C)III and IV
D)III,IV,V
E)all can be dehydrated

A)II,IV,V
B)I,II,III
C)III and IV
D)III,IV,V
E)all can be dehydrated

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
59
What is the major product for the following reaction? 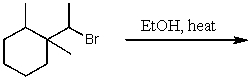
A)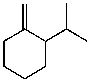
B)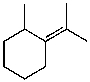
C)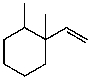
D)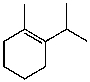
E)More than one of these choices.
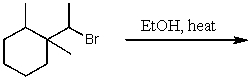
A)

B)

C)

D)

E)More than one of these choices.

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
60
Which compound would be the major product? 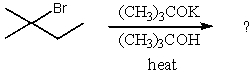
A)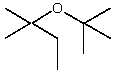
B)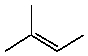
C)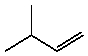
D)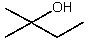
E)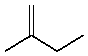
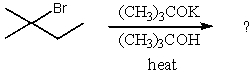
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
61
What is the major product of the reaction of the following reaction? 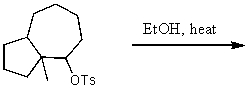
A)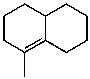
B)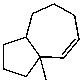
C)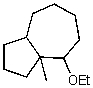
D)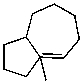
E)None of these choices.
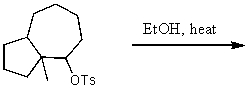
A)

B)

C)

D)

E)None of these choices.

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
62
What will be the major product of the following reaction? 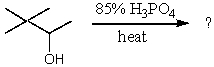
A)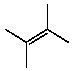
B)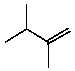
C)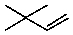
D)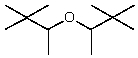
E)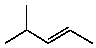
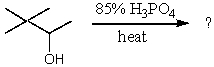
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
63
Which reaction would not result in alkylation of the acetylide anion as the major products?
A)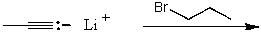
B)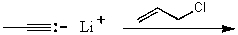
C)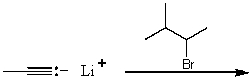
D)
E)None of these choices.
A)
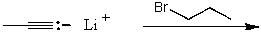
B)
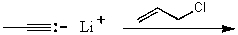
C)

D)

E)None of these choices.

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following methods could be used to synthesize 4,4-dimethyl-2-hexyne?
A)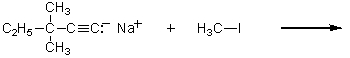
B)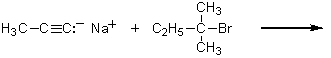
C)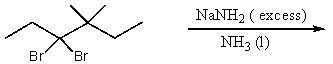
D)More than one of these choices.
E)None of these choices.
A)
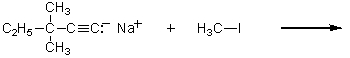
B)
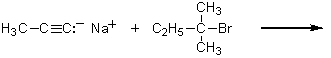
C)
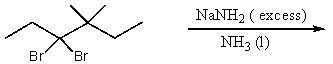
D)More than one of these choices.
E)None of these choices.

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following reactions would yield 2-pentyne?
A)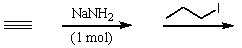
B)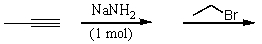
C)
D)
E)
A)
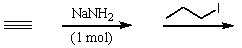
B)
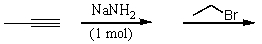
C)

D)

E)
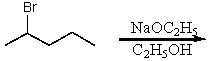

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
66
Which reaction would yield 2-butyne?
A)CH3C C:-Na+ + CH3Br
B)CH3CH2Br + HC C:-Na+
C)CH3:-Na+ + HC CCH3
D)More than one of these choices.
E)None of these choices.
A)CH3C C:-Na+ + CH3Br
B)CH3CH2Br + HC C:-Na+
C)CH3:-Na+ + HC CCH3
D)More than one of these choices.
E)None of these choices.

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
67
What is the major product for the following reaction? 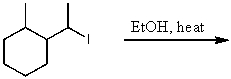
A)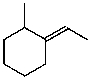
B)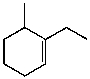
C)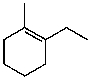
D)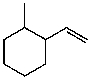
E)None of these choices.
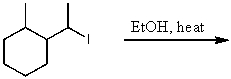
A)

B)

C)

D)

E)None of these choices.

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
68
Which reaction conditions would not yield 2-butyne from 1-propyne?
A)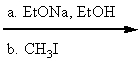
B)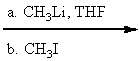
C)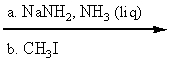
D)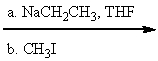
E)More than one of these choices.
A)
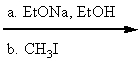
B)
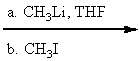
C)
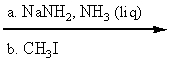
D)
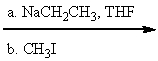
E)More than one of these choices.

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
69
What is the major product(s)of the reaction of the following reaction sequence? 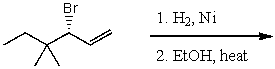
A)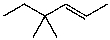
B)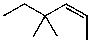
C)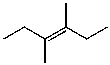
D)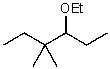
E)A mixture of two of these choices.
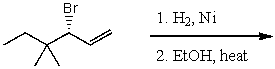
A)

B)

C)

D)

E)A mixture of two of these choices.

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
70
A compound X with the formula C7H10 undergoes catalytic hydrogenation to produce a compound Y with the formula C7H14. What could be true of X?
A)X might have one triple bond and one ring.
B)X might have two double bonds and one ring.
C)X might have one double bond and two rings.
D)X might have one double bond and one triple bond.
E)More than one of these choices.
A)X might have one triple bond and one ring.
B)X might have two double bonds and one ring.
C)X might have one double bond and two rings.
D)X might have one double bond and one triple bond.
E)More than one of these choices.

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
71
What is the major product of the reaction of the following reaction? 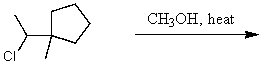
A)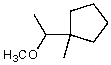
B)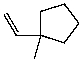
C)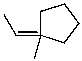
D)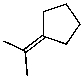
E)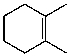
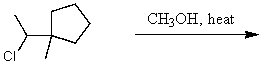
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
72
An unknown compound,B,has the molecular formula C7H12.On catalytic hydrogenation 1 mol of B absorbs 2 mol of hydrogen and yields 2-methylhexane.B has significant IR absorption band at about 3300 and 2200 cm-1.Which compound best represents B?
A)3-methyl-1-hexyne
B)5-methyl-2-hexyne
C)5-methyl-1,3-hexadiene
D)5-methyl-1-hexyne
E)2-methyl-1,5-hexadiene
A)3-methyl-1-hexyne
B)5-methyl-2-hexyne
C)5-methyl-1,3-hexadiene
D)5-methyl-1-hexyne
E)2-methyl-1,5-hexadiene

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
73
What is the major product of the reaction of the following reaction? 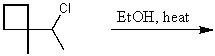
A)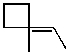
B)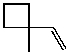
C)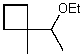
D)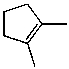
E)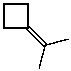
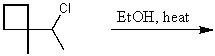
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
74
A compound,C6H10,which reacts with 2 mol of hydrogen over a platinum catalyst and which shows an IR absorption band at approximately 3300 cm-1 could be: 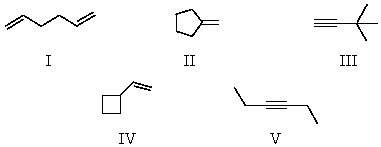
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
75
What is the major product for the following reaction? 
A)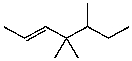
B)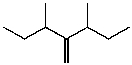
C)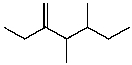
D)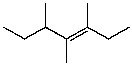
E)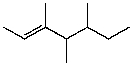
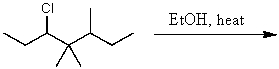
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
76
Which reaction would not primarily proceed via an SN2 mechanism?
A)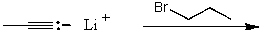
B)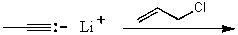
C)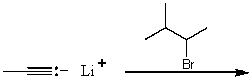
D)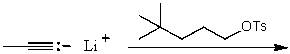
E)All of them proceed via SN2
A)
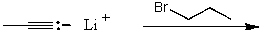
B)
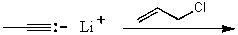
C)

D)

E)All of them proceed via SN2

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
77
Which would be the major product of the following reaction sequence? 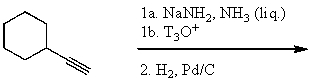
A)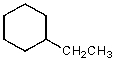
B)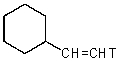
C)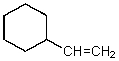
D)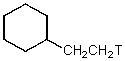
E)None of these choices.
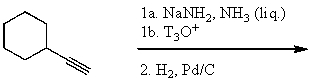
A)

B)

C)

D)

E)None of these choices.

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following reactions would yield 3,3-dimethyl-1-butene in a reasonable percentage yield (i.e.,greater than 50%)?
A)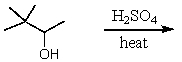
B)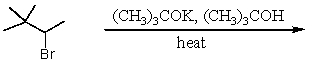
C)

D)All of these choices.
E)Only two of these choices.
A)
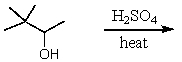
B)
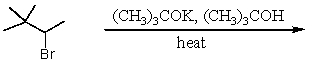
C)

D)All of these choices.
E)Only two of these choices.

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
79
Which statement is/are true about acetylide anions?
A)The alkylated product is poor with secondary alkyl halides.
B)Primary alkyl halides are best suited for alkylation.
C)In the presence of tertiary alkyl halides,the acetylide anion acts as base to give an elimination product.
D)Only two of these statements are true.
E)All of the statements are true.
A)The alkylated product is poor with secondary alkyl halides.
B)Primary alkyl halides are best suited for alkylation.
C)In the presence of tertiary alkyl halides,the acetylide anion acts as base to give an elimination product.
D)Only two of these statements are true.
E)All of the statements are true.

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck
80
Which alkene would you expect to be the major product of the following dehydration? 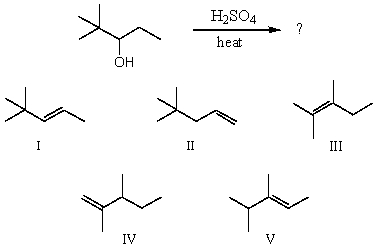
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 220 flashcards in this deck.
Unlock Deck
k this deck



