Deck 6: Nucleophilic Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/91
Play
Full screen (f)
Deck 6: Nucleophilic Reactions
1
An increase in the temperature at which a reaction is carried out increases
A)the collision frequency.
B)the fraction of molecules with proper orientation.
C)the fraction of molecules with energy greater than Eact.
D)More than one of these choices.
E)None of these choices.
A)the collision frequency.
B)the fraction of molecules with proper orientation.
C)the fraction of molecules with energy greater than Eact.
D)More than one of these choices.
E)None of these choices.
More than one of these choices.
2
Select the potential energy diagram that represents a two-step endothermic (endergonic)reaction. 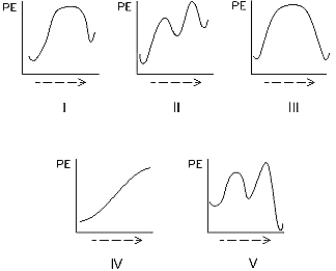
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V
II
3
Consider the SN2 reaction of butyl bromide with OH- ion. CH3CH2CH2CH2Br + OH- CH3CH2CH2CH2OH + Br-
Assuming no other changes,what effect on the rate would result from simultaneously doubling the concentrations of both butyl bromide and OH- ion?
A)No effect.
B)It would double the rate.
C)It would triple the rate.
D)It would increase the rate four times.
E)It would increase the rate six times.
Assuming no other changes,what effect on the rate would result from simultaneously doubling the concentrations of both butyl bromide and OH- ion?
A)No effect.
B)It would double the rate.
C)It would triple the rate.
D)It would increase the rate four times.
E)It would increase the rate six times.
It would increase the rate four times.
4
The rate equation for a nucleophilic substitution reaction of a tertiary alkyl bromide (R-Br)with I- ion would be:
A)Rate = k [RBr]
B)Rate = k [I-]
C)Rate = k [RBr][I-]
D)Rate = k [RBr]2[I-]
E)Rate = k [RBr][I-]2
A)Rate = k [RBr]
B)Rate = k [I-]
C)Rate = k [RBr][I-]
D)Rate = k [RBr]2[I-]
E)Rate = k [RBr][I-]2

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
5
Consider the SN2 reaction of 2-iodopentane with CH3CO2- ion. 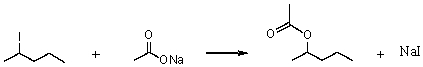
Assuming no other changes,what effect on the rate would result from simultaneously doubling the concentrations of both 2-iodopentane and sodium acetate?
A)No effect.
B)It would double the rate.
C)It would triple the rate.
D)It would increase the rate four times.
E)It would increase the rate six times.

Assuming no other changes,what effect on the rate would result from simultaneously doubling the concentrations of both 2-iodopentane and sodium acetate?
A)No effect.
B)It would double the rate.
C)It would triple the rate.
D)It would increase the rate four times.
E)It would increase the rate six times.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
6
The reaction, 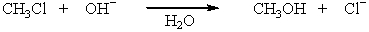 has the following thermodynamic values at 27.0 ºC: H = -75.3 kJ mol-1; S = 54.4 J K-1 mol-1.What is the value of G for this reaction?
has the following thermodynamic values at 27.0 ºC: H = -75.3 kJ mol-1; S = 54.4 J K-1 mol-1.What is the value of G for this reaction?
A)-73.8 kJ mol-1
B)-76.8 kJ mol-1
C)-59.0 kJ mol-1
D)+91.6 kJ mol-1
E)-91.6 kJ mol-1
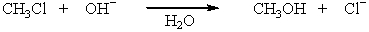 has the following thermodynamic values at 27.0 ºC: H = -75.3 kJ mol-1; S = 54.4 J K-1 mol-1.What is the value of G for this reaction?
has the following thermodynamic values at 27.0 ºC: H = -75.3 kJ mol-1; S = 54.4 J K-1 mol-1.What is the value of G for this reaction?A)-73.8 kJ mol-1
B)-76.8 kJ mol-1
C)-59.0 kJ mol-1
D)+91.6 kJ mol-1
E)-91.6 kJ mol-1

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
7
If the rate of reaction of [0.1 M] sodium cyanide with [0.1 M] 2-bromo-2-methylpropane is 1.2 x 10-3 M/s,what would be the effect on the overall rate if the concentration of sodium cyanide is increased to [0.2 M] and the concentration of the alkyl bromide is decreased to [0.05 M]?
A)the rate will increase by a factor of 2
B)the rate will decrease by a factor of 2
C)the rate will increase by a factor of 10
D)the rate will decrease by a factor of 10
E)the rate will remain unchanged
A)the rate will increase by a factor of 2
B)the rate will decrease by a factor of 2
C)the rate will increase by a factor of 10
D)the rate will decrease by a factor of 10
E)the rate will remain unchanged

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
8
The difference in the bond energies of reactants and the transition state of a reaction is designated by the notation:
A)" H "
B)" H‡"
C)" G "
D)" G‡"
E)" S‡"
A)" H "
B)" H‡"
C)" G "
D)" G‡"
E)" S‡"

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
9
The hybridization state of the charged carbon in a carbocation is ___.
A)sp4
B)sp3
C)sp2
D)sp
E)s
A)sp4
B)sp3
C)sp2
D)sp
E)s

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
10
The rate equation for a nucleophilic substitution reaction of a secondary alkyl chloride (R-Cl)with I- ion would be:
A)Rate = k [RCl]
B)Rate = k [I-]
C)Rate = k [RCl][I-]
D)Rate = k [RCl]2[I-]
E)Rate = k [RCl][I-]2
A)Rate = k [RCl]
B)Rate = k [I-]
C)Rate = k [RCl][I-]
D)Rate = k [RCl]2[I-]
E)Rate = k [RCl][I-]2

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
11
Which will be true for any actual or potential nucleophilic substitution reaction?
A)" H is positive."
B)" H is negative."
C)" G‡ is positive."
D)" G is positive."
E)" G is negative."
A)" H is positive."
B)" H is negative."
C)" G‡ is positive."
D)" G is positive."
E)" G is negative."

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
12
A true statement about the transition state(s)of an SN2 reaction is:
A)The two transition states are of unequal energy.
B)The transition states precede and follow an unstable reaction intermediate.
C)The single transition state represents the point of maximum free energy of the reaction.
D)Existence of this transition state implies an exothermic reaction.
E)The transition state will always have a net charge of -1.
A)The two transition states are of unequal energy.
B)The transition states precede and follow an unstable reaction intermediate.
C)The single transition state represents the point of maximum free energy of the reaction.
D)Existence of this transition state implies an exothermic reaction.
E)The transition state will always have a net charge of -1.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
13
Consider the reaction of 2-chloro-2-methylpentane with sodium iodide. 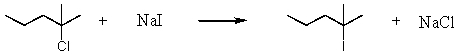 Assuming no other changes,how would it affect the rate if one simultaneously doubled the concentration of 2-chloro-2-methylpentane and sodium iodide?
Assuming no other changes,how would it affect the rate if one simultaneously doubled the concentration of 2-chloro-2-methylpentane and sodium iodide?
A)No effect.
B)It would double the rate.
C)It would triple the rate.
D)It would quadruple the rate.
E)It would increase the rate five times.
 Assuming no other changes,how would it affect the rate if one simultaneously doubled the concentration of 2-chloro-2-methylpentane and sodium iodide?
Assuming no other changes,how would it affect the rate if one simultaneously doubled the concentration of 2-chloro-2-methylpentane and sodium iodide?A)No effect.
B)It would double the rate.
C)It would triple the rate.
D)It would quadruple the rate.
E)It would increase the rate five times.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
14
The major product of the following reaction would be: 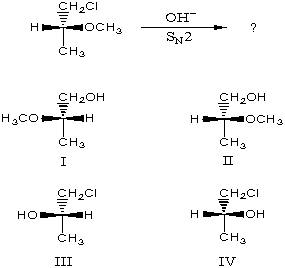
A)I
B)II
C)III
D)IV
E)An equimolar mixture of I and II.

A)I
B)II
C)III
D)IV
E)An equimolar mixture of I and II.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
15
Select the potential energy diagram that represents a single-step endothermic (endergonic)reaction. 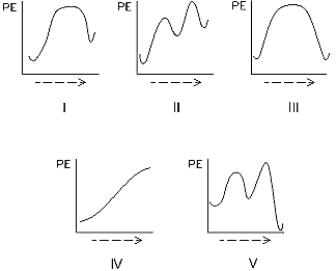
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
16
Increasing the temperature of a chemical reaction usually increases greatly the rate of the reaction.The most important reason for this is that increasing the temperature
A)increases the collision frequency.
B)decreases the probability factor.
C)increases the fraction of collisions with energy greater than Eact.
D)decreases the energy of activation.
E)makes the reaction more exothermic.
A)increases the collision frequency.
B)decreases the probability factor.
C)increases the fraction of collisions with energy greater than Eact.
D)decreases the energy of activation.
E)makes the reaction more exothermic.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
17
What product(s)would you expect to obtain from the following SN2 reaction? 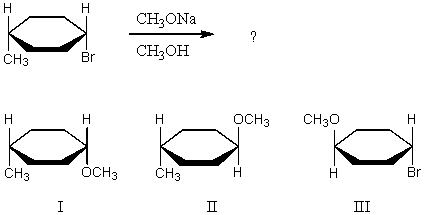
A)I
B)II
C)An equimolar mixture of I and II.
D)III
E)None of these choices.

A)I
B)II
C)An equimolar mixture of I and II.
D)III
E)None of these choices.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
18
Select the rate law for the following reaction,e.g., CH3CH2CH2CHBrCH3 + OH- CH3CH2CH2CHOHCH3 + Br -
( RBr )
A)Rate = k [RBr]
B)Rate = k [RBr] [OH-]
C)Rate = k [RBr]2 [OH-]
D)Rate = k [RBr] [OH-]2
E)Rate = k [RBr]2 [OH-]2
( RBr )
A)Rate = k [RBr]
B)Rate = k [RBr] [OH-]
C)Rate = k [RBr]2 [OH-]
D)Rate = k [RBr] [OH-]2
E)Rate = k [RBr]2 [OH-]2

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
19
Select the potential energy diagram that represents an exothermic (exergonic)reaction. 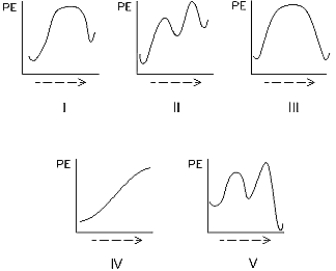
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
20
Consider the SN2 reaction of 1-chloro-5-methylhexane with CN- ion. 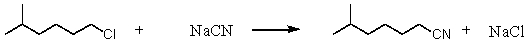
Assuming no other changes,what effect on the rate would result from simultaneously doubling the concentrations of both 1-chloro-5-methylhexane and NaCN?
A)No effect.
B)It would double the rate.
C)It would triple the rate.
D)It would increase the rate four times.
E)It would increase the rate six times.
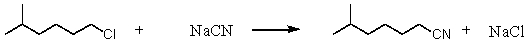
Assuming no other changes,what effect on the rate would result from simultaneously doubling the concentrations of both 1-chloro-5-methylhexane and NaCN?
A)No effect.
B)It would double the rate.
C)It would triple the rate.
D)It would increase the rate four times.
E)It would increase the rate six times.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
21
Ambident nucleophiles are ones which can react with a substrate at either of two nucleophilic sites.Which of the following is not an ambident nucleophile? 
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
22
Rank the following in terms of nucleophilic strength: 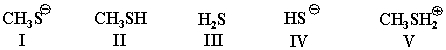
A)I>II>III>IV>V
B)V>IV>III>II>I
C)I>IV>II>III>V
D)V>I>IV>II>III
E)I>II>IV>V>III
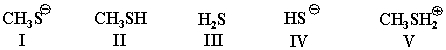
A)I>II>III>IV>V
B)V>IV>III>II>I
C)I>IV>II>III>V
D)V>I>IV>II>III
E)I>II>IV>V>III

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
23
Which is the weakest nucleophile in polar aprotic solvents?
A)I-
B)Br-
C)Cl-
D)F-
E)All of these choices are equally strong nucleophiles,regardless of the type of solvent used.
A)I-
B)Br-
C)Cl-
D)F-
E)All of these choices are equally strong nucleophiles,regardless of the type of solvent used.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
24
An increase in the kinetic energy of reacting molecules results in
A)a decrease in reaction rate.
B)an increase in the probability factor.
C)a decrease in the probability factor.
D)an increase in the reaction rate.
E)no changes.
A)a decrease in reaction rate.
B)an increase in the probability factor.
C)a decrease in the probability factor.
D)an increase in the reaction rate.
E)no changes.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
25
Reaction of (R)-2-chloro-4-methylhexane with excess NaI in acetone gives racemic 2-iodo-4-methylhexane.What is the explanation that best describes this transformation?
A)an SN2 reaction has occurred with inversion of configuration
B)racemization followed by an SN2 attack
C)an SNI reaction has taken over resulting in inversion of configuration
D)an SN1 reaction has occurred due to carbocation formation
E)an SN1 reaction followed by an SN2 "backside" attack
A)an SN2 reaction has occurred with inversion of configuration
B)racemization followed by an SN2 attack
C)an SNI reaction has taken over resulting in inversion of configuration
D)an SN1 reaction has occurred due to carbocation formation
E)an SN1 reaction followed by an SN2 "backside" attack

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
26
Which alkyl halide would you expect to undergo an SN2 reaction most slowly?
A)1-bromohexane
B)1-bromo-2-methylpentane
C)1-bromo-3-methylpentane
D)1-bromo-4-methylpentane
E)1-bromo-2,2-dimethylbutane
A)1-bromohexane
B)1-bromo-2-methylpentane
C)1-bromo-3-methylpentane
D)1-bromo-4-methylpentane
E)1-bromo-2,2-dimethylbutane

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
27
Which SN2 reaction would you expect to take place most rapidly? Assume that the concentrations of the reactants and the temperature are the same in each instance.
A)
B)
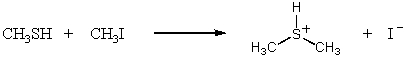
C)
D)
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
28
Identify the nucleophile in the following reaction: 2 H2O + RX ROH + H3O+ + X-
A)X-
B)H3O+
C)ROH
D)H2O
E)RX
A)X-
B)H3O+
C)ROH
D)H2O
E)RX

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
29
The p orbital of the charged carbon in the isopropyl cation,(CH3)2CH+,contains how many electrons?
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
30
Which is not a polar aprotic solvent?
A)
B)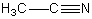
C)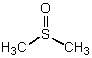
D)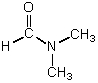
E)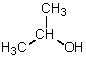
A)

B)
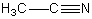
C)

D)

E)


Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
31
Which is the weakest nucleophile in polar protic solvents?
A)I-
B)Br-
C)Cl-
D)F-
E)All of these choices are equally strong nucleophiles,regardless of the type of solvent used.
A)I-
B)Br-
C)Cl-
D)F-
E)All of these choices are equally strong nucleophiles,regardless of the type of solvent used.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
32
Which alkyl chloride,though primary,is essentially unreactive in SN2 reactions?
A)
B)
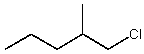
C)
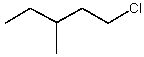
D)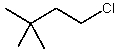
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
33
Which ion is the strongest nucleophile in aqueous solution?
A)F-
B)Cl-
C)Br-
D)I-
E)All of these choices are equally strong.
A)F-
B)Cl-
C)Br-
D)I-
E)All of these choices are equally strong.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
34
Consider the substitution reaction that takes place when (R)-3-bromo-3-methylhexane is treated with methanol.Which of the following would be true?
A)The reaction would take place only with inversion of configuration at the stereogenic center.
B)The reaction would take place only with retention of configuration at the stereogenic center.
C)The reaction would take place with racemization.
D)No reaction would take place.
E)The alkyl halide does not possess a stereogenic center.
A)The reaction would take place only with inversion of configuration at the stereogenic center.
B)The reaction would take place only with retention of configuration at the stereogenic center.
C)The reaction would take place with racemization.
D)No reaction would take place.
E)The alkyl halide does not possess a stereogenic center.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following is not a nucleophile?
A)H2O
B)CH3O-
C)NH3
D)NH4+
E)All are nucleophiles.
A)H2O
B)CH3O-
C)NH3
D)NH4+
E)All are nucleophiles.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following alkyl bromide isomers would most readily undergo an SN2 reaction?
A)bromocyclobutane
B)4-bromo-1-butene
C)3-bromo-1-butene
D)1-bromo-1-butene
E)All will react at the same rate.
A)bromocyclobutane
B)4-bromo-1-butene
C)3-bromo-1-butene
D)1-bromo-1-butene
E)All will react at the same rate.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
37
Which is the strongest nucleophile?
A)OH-
B)CH3CH2O-
C)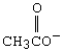
D)CH3CH2OH
E)H2O
A)OH-
B)CH3CH2O-
C)
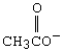
D)CH3CH2OH
E)H2O

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
38
Which alkyl halide would be most reactive in an SN1 reaction? 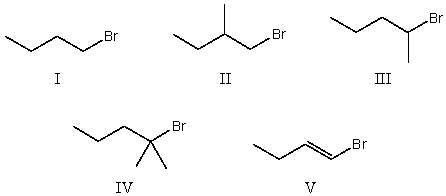
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
39
Which is a polar aprotic solvent?
A)2-methylhexane
B)CCl4
C)NH3(l)
D)CH3CH2CH2OCH2CH2CH3
E)2-methyl-2-propanol
A)2-methylhexane
B)CCl4
C)NH3(l)
D)CH3CH2CH2OCH2CH2CH3
E)2-methyl-2-propanol

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
40
Identify the nucleophile in the following reaction: 2R'OH + RX ROR' + [R'OH2]+ + X-
A)X-
B)[R'OH2]-
C)ROR'
D)R'OH
E)RX
A)X-
B)[R'OH2]-
C)ROR'
D)R'OH
E)RX

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following would be most reactive in an SN2 reaction?
A)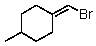
B)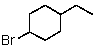
C)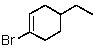
D)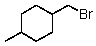
E)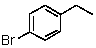
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
42
Identify the leaving group in the following reaction. 
A)C6H5S-
B)Na+
C)CH3CH2I
D)C6H5SCH2CH3
E)I-

A)C6H5S-
B)Na+
C)CH3CH2I
D)C6H5SCH2CH3
E)I-

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
43
Which SN2 reaction would take place most rapidly?
A)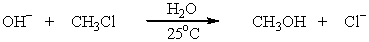
B)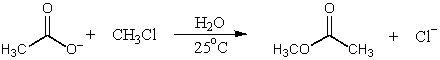
C)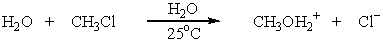
D)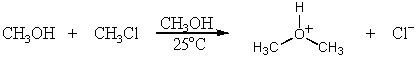
E)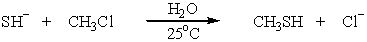
A)
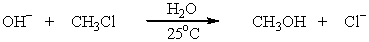
B)

C)
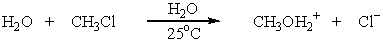
D)
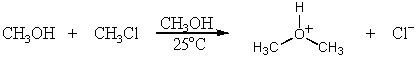
E)
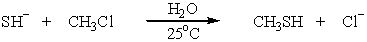

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following nucleophiles will react the fastest with 1-bromoptopane in ethanol?
A)SH-
B)OH-
C)H2O
D)H2S
E)All react at about the same rate.
A)SH-
B)OH-
C)H2O
D)H2S
E)All react at about the same rate.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following is a feasible substitution reaction?
A)CH3CH2Cl + NaBr CH3CH2Br + NaCl
B)CH3CH3 + NaCN CH3CH2CN + NaH
C)CH3CH2Cl + NaOH CH2=CH2 + H2O + NaCl
D)More than one of these choices.
E)None of these choices.
A)CH3CH2Cl + NaBr CH3CH2Br + NaCl
B)CH3CH3 + NaCN CH3CH2CN + NaH
C)CH3CH2Cl + NaOH CH2=CH2 + H2O + NaCl
D)More than one of these choices.
E)None of these choices.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
46
Which SN2 reaction will occur most rapidly in aqueous acetone solution? Assume concentrations and temperature are the same in each instance.
A)HO- + CH3-Cl CH3OH + Cl-
B)HO- + CH3CH2-Cl CH3CH2OH + Cl-
C)HO- + (CH3)2CH-Cl (CH3)2CHOH + Cl-
D)HO- + (CH3)3C-Cl (CH3)3COH + Cl-
E)HO- + (CH3)3CCH2-Cl (CH3)3CCH2OH + Cl-
A)HO- + CH3-Cl CH3OH + Cl-
B)HO- + CH3CH2-Cl CH3CH2OH + Cl-
C)HO- + (CH3)2CH-Cl (CH3)2CHOH + Cl-
D)HO- + (CH3)3C-Cl (CH3)3COH + Cl-
E)HO- + (CH3)3CCH2-Cl (CH3)3CCH2OH + Cl-

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
47
Which SN2 reaction will occur most rapidly in a mixture of water and ethanol?
A)I- + CH3CH2-Br CH3CH2-I + Br-
B)I- + CH3CH2-Cl CH3CH2-I + Cl-
C)I- + CH3CH2-F CH3CH2-I + F-
D)Br- + CH3CH2-Cl CH3CH2-Br + Cl-
E)Br- + CH3CH2-F CH3CH2-Br + F-
A)I- + CH3CH2-Br CH3CH2-I + Br-
B)I- + CH3CH2-Cl CH3CH2-I + Cl-
C)I- + CH3CH2-F CH3CH2-I + F-
D)Br- + CH3CH2-Cl CH3CH2-Br + Cl-
E)Br- + CH3CH2-F CH3CH2-Br + F-

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following is the poorest leaving group?
A)H-
B)CH3O-
C)H2O
D)OH-
E)NH2-
A)H-
B)CH3O-
C)H2O
D)OH-
E)NH2-

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following solvents will best promote a nucleophilic reaction between NaCN and 1-bromopropane?
A)H2O/MeOH mixture
B)H2O
C)MeCN
D)HF(aq)
E)running with no solvent
A)H2O/MeOH mixture
B)H2O
C)MeCN
D)HF(aq)
E)running with no solvent

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
50
The relative nucleophilicities of species do not necessarily parallel the relative basicities of the same species because
A)not all nucleophiles are bases,and vice versa.
B)experimental measurements of sufficient accuracy are not available to make the comparisons.
C)nucleophilicity is a thermodynamic matter; basicity is a matter of kinetics.
D)basicity is a thermodynamic matter; nucleophilicity is a matter of kinetics.
E)Actually,the relative values do parallel one another.
A)not all nucleophiles are bases,and vice versa.
B)experimental measurements of sufficient accuracy are not available to make the comparisons.
C)nucleophilicity is a thermodynamic matter; basicity is a matter of kinetics.
D)basicity is a thermodynamic matter; nucleophilicity is a matter of kinetics.
E)Actually,the relative values do parallel one another.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following nucleophiles will react the fastest with tert-butyl bromide in ethanol?
A)F-
B)Br-
C)I-
D)Cl-
E)All react at about the same rate.
A)F-
B)Br-
C)I-
D)Cl-
E)All react at about the same rate.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
52
Which alkyl halide would you expect to react most slowly when heated in aqueous solution?
A)(CH3)3C-F
B)(CH3)3C-Cl
C)(CH3)3C-Br
D)(CH3)3C-I
E)All of these choices would react at the same rate.
A)(CH3)3C-F
B)(CH3)3C-Cl
C)(CH3)3C-Br
D)(CH3)3C-I
E)All of these choices would react at the same rate.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
53
Identify the leaving group in the following reaction. 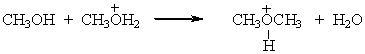
A)CH3OH
B)CH3OH2+
C)CH3OCH3
D)H2O
E)None of these choices.

A)CH3OH
B)CH3OH2+
C)CH3OCH3
D)H2O
E)None of these choices.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
54
Which is the most reactive nucleophile in DMF (structure shown below)? 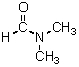
A)F-
B)Cl-
C)Br-
D)I-
E)All of these choices are equally reactive.

A)F-
B)Cl-
C)Br-
D)I-
E)All of these choices are equally reactive.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
55
Which ion is the strongest nucleophile in an aprotic solvent such as dimethylsulfoxide?
A)I-
B)Br-
C)Cl-
D)F-
E)All of these choices are equal.
A)I-
B)Br-
C)Cl-
D)F-
E)All of these choices are equal.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
56
Which nucleophilic substitution reaction would be unlikely to occur?
A)HO- + CH3CH2-I CH3CH2-OH + I-
B)I- + CH3CH2-H CH3CH2-I + H-
C)CH3S- + CH3-Br CH3S-CH3 + Br-
D)All of these choices would be unlikely to occur.
E)None of these choices would be unlikely to occur.
A)HO- + CH3CH2-I CH3CH2-OH + I-
B)I- + CH3CH2-H CH3CH2-I + H-
C)CH3S- + CH3-Br CH3S-CH3 + Br-
D)All of these choices would be unlikely to occur.
E)None of these choices would be unlikely to occur.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
57
Considering the relative solvation of reactants and the transition states of substitution reactions of these reactants,predict which general type of reaction would be most favored by the use of a polar solvent.
A)Y: + RX RY+ + X:-
B)Y:- + RX RY + X:-
C)Y: + RX+ RY+ + X:
D)Y:- + RX+ RY + X:
E)RX+ R+ + X:
A)Y: + RX RY+ + X:-
B)Y:- + RX RY + X:-
C)Y: + RX+ RY+ + X:
D)Y:- + RX+ RY + X:
E)RX+ R+ + X:

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
58
Which of these species,acting in a protic solvent,exhibits greater nucleophilic activity than expected on the basis of its basicity?
A)OH-
B)CH3O-
C)SH-
D)Cl-
E)H2O
A)OH-
B)CH3O-
C)SH-
D)Cl-
E)H2O

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following is not true concerning the strength of a nucleophile?
A)Nucleophilicity may not parallel basicity.
B)Negatively charged nucleophiles are always more reactive than their conjugate acids.
C)The greater the strength of a nucleophile,the faster an SN2 reaction will occur.
D)Strong bases are always good nucleophiles
E)None of these answer choices are correct.
A)Nucleophilicity may not parallel basicity.
B)Negatively charged nucleophiles are always more reactive than their conjugate acids.
C)The greater the strength of a nucleophile,the faster an SN2 reaction will occur.
D)Strong bases are always good nucleophiles
E)None of these answer choices are correct.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following is not a good leaving group?
A)C2H5O-
B)Cl-
C)Isup>-
D)CH3CO2-
E)All of these choices are good leaving groups.
A)C2H5O-
B)Cl-
C)Isup>-
D)CH3CO2-
E)All of these choices are good leaving groups.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
61
In the SN2 reaction,the unstable arrangement of atoms in which both the nucleophile and the leaving group are partially bonded to the same carbon atom is called the ___.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
62
SN2 reactions of the type,Nu- + RL Nu-R + L-, are favored
A)when tertiary substrates are used.
B)by using a high concentration of the nucleophile.
C)by using a solvent of high polarity.
D)by the use of weak nucleophiles.
E)by none of these choices.
A)when tertiary substrates are used.
B)by using a high concentration of the nucleophile.
C)by using a solvent of high polarity.
D)by the use of weak nucleophiles.
E)by none of these choices.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
63
SN1 reactions of the following type: Nu:- + R-X R-Nu + :X-
Are favored
A)by the use of tertiary substrates (as opposed to primary or secondary substrates).
B)by increasing the concentration of the nucleophile.
C)by increasing the polarity of the solvent.
D)by use of a weak nucleophile.
E)by the use of tertiary substrates (as opposed to primary or secondary substrates)and by use of a weak nucleophile.
Are favored
A)by the use of tertiary substrates (as opposed to primary or secondary substrates).
B)by increasing the concentration of the nucleophile.
C)by increasing the polarity of the solvent.
D)by use of a weak nucleophile.
E)by the use of tertiary substrates (as opposed to primary or secondary substrates)and by use of a weak nucleophile.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
64
As we go down Group 7A of the periodic table,the size of the halogen atom increases; accordingly,the carbon-halogen bond length gets ___ and the bond strength gets ___.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
65
In order for colliding species to react,they must ___ and ___.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
66
Racemic mixtures form in SN1 reactions when leaving group departure results in a loss of chirality followed by subsequent attack of the nucleophile.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following statements is (are)true of an SN2 reaction of (R)-2-bromobutane with hydroxide ion?
A)Doubling the hydroxide ion concentration would double the rate of the reaction.(Assume that all other experimental conditions are unchanged.)
B)The major product would be (S)-2-butanol.
C)Doubling the concentration of (R)-2-bromobutane would double the rate of the reaction.(Assume that all other experimental conditions are unchanged.)
D)All of these choices.
E)Two of these choices.
A)Doubling the hydroxide ion concentration would double the rate of the reaction.(Assume that all other experimental conditions are unchanged.)
B)The major product would be (S)-2-butanol.
C)Doubling the concentration of (R)-2-bromobutane would double the rate of the reaction.(Assume that all other experimental conditions are unchanged.)
D)All of these choices.
E)Two of these choices.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
68
SN1 reactions of the type,Nu- + RL Nu-R + L-,are favored
A)when tertiary substrates are used.
B)by using a high concentration of the nucleophile.
C)when L- is a strong base.
D)by use of a non-polar solvent.
E)by none of these choices.
A)when tertiary substrates are used.
B)by using a high concentration of the nucleophile.
C)when L- is a strong base.
D)by use of a non-polar solvent.
E)by none of these choices.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
69
What final product is likely to be obtained through the following series of reactions? 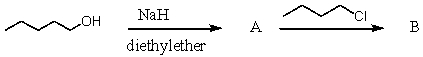


Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following statements is (are)true of SN1 reactions of alkyl halides in general?
A)The rate of an SN1 reaction depends on the concentration of the alkyl halide.
B)The rate of an SN1 reaction depends on the concentration of the nucleophile.
C)SN1 reactions of alkyl halides are favored by polar solvents.
D)The rate of an SN1 reaction depends on the concentration of the alkyl halide and SN1 reactions of alkyl halides are favored by polar solvents.
E)The rate of an SN1 reaction depends on the concentration of the alkyl halide and the concentration of the nucleophile,and SN1 reactions of alkyl halides are favored by polar solvents.
A)The rate of an SN1 reaction depends on the concentration of the alkyl halide.
B)The rate of an SN1 reaction depends on the concentration of the nucleophile.
C)SN1 reactions of alkyl halides are favored by polar solvents.
D)The rate of an SN1 reaction depends on the concentration of the alkyl halide and SN1 reactions of alkyl halides are favored by polar solvents.
E)The rate of an SN1 reaction depends on the concentration of the alkyl halide and the concentration of the nucleophile,and SN1 reactions of alkyl halides are favored by polar solvents.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
71
SN1 reactions of the following type: Nu:- + R-X R-Nu + :X-
Are favored
A)by the use of tertiary substrates (as opposed to primary or secondary substrates).
B)by increasing the concentration of the nucleophile.
C)by increasing the polarity of the solvent.
D)by use of a strong base.
E)by more than one of these choices.
Are favored
A)by the use of tertiary substrates (as opposed to primary or secondary substrates).
B)by increasing the concentration of the nucleophile.
C)by increasing the polarity of the solvent.
D)by use of a strong base.
E)by more than one of these choices.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following statements is (are)true of SN1 reactions of alkyl halides in general?
A)The rate of an SN1 reaction depends on the concentration of the alkyl halide.
B)The rate of an SN1 reaction depends on the concentration of the nucleophile.
C)SN1 reactions of alkyl halides occur faster in polar aprotic solvents (compared to protic solvents).
D)The rate of an SN1 reaction depends on the concentration of the alkyl halide and SN1 reactions of alkyl halides occur faster in polar aprotic solvents (compared to protic solvents).
E)The rate of an SN1 reaction depends on the concentration of the alkyl halide and the concentration of the nucleophile,and SN1 reactions of alkyl halides occur faster in polar aprotic solvents (compared to protic solvents).
A)The rate of an SN1 reaction depends on the concentration of the alkyl halide.
B)The rate of an SN1 reaction depends on the concentration of the nucleophile.
C)SN1 reactions of alkyl halides occur faster in polar aprotic solvents (compared to protic solvents).
D)The rate of an SN1 reaction depends on the concentration of the alkyl halide and SN1 reactions of alkyl halides occur faster in polar aprotic solvents (compared to protic solvents).
E)The rate of an SN1 reaction depends on the concentration of the alkyl halide and the concentration of the nucleophile,and SN1 reactions of alkyl halides occur faster in polar aprotic solvents (compared to protic solvents).

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
73
Increasing the temperature of a reaction will speed up the overall rate as this will increase the energy of activation for reaction.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
74
Which nucleophilic substitution reaction is not likely to occur?
A)I-+ CH3CH2-Cl CH3CH2-I + Cl-
B)I- + CH3CH2-Br CH3CH2-I + Br-
C)I- + CH3CH2-OH CH3CH2-I + OH-
D)CH3O-+ CH3CH2-Br CH3CH2-OCH3 + Br-
E)OH-+ CH3CH2-Cl CH3CH2-OH + Cl-
A)I-+ CH3CH2-Cl CH3CH2-I + Cl-
B)I- + CH3CH2-Br CH3CH2-I + Br-
C)I- + CH3CH2-OH CH3CH2-I + OH-
D)CH3O-+ CH3CH2-Br CH3CH2-OCH3 + Br-
E)OH-+ CH3CH2-Cl CH3CH2-OH + Cl-

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
75
Draw the potential energy diagram that represents an exothermic reaction between a tertiary alkyl halide and methanol.Briefly explain your rationale.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
76
When 5-bromo-1-pentanol is treated with sodium hydride in diethyl ether,the product is analyzed to be C5H10O.Propose a likely structure for this product,suggesting a reasonable mechanistic pathway for its formation.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
77
In a highly exergonic SN2 reaction we can assume the transition state is similar to the products formed in the reaction.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
78
A nucleophile in an SN2 reaction must approach from the ___ of the carbon atom attached to the leaving group since the electrons in the nucleophile's HOMO begins to overlap with the ___ of the carbon atom attached to the leaving group.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
79
Which nucleophilic substitution reaction would be likely to occur (although probably not in excellent yield,due to competing elimination)?
A)HO- + CH3CH2CH2CHICH3 CH3 CH2CH2CHOHCH3 + I-
B)Cl-+ CH3CH2CH2CH2-OH CH3CH2CH2CH2-Cl + OH-
C)CH3S- + CH3CH2CH2-OCH3 CH3S-CH2CH2CH3 + OCH3---
D)All of these choices are likely to occur.
E)None of these choices are likely to occur.
A)HO- + CH3CH2CH2CHICH3 CH3 CH2CH2CHOHCH3 + I-
B)Cl-+ CH3CH2CH2CH2-OH CH3CH2CH2CH2-Cl + OH-
C)CH3S- + CH3CH2CH2-OCH3 CH3S-CH2CH2CH3 + OCH3---
D)All of these choices are likely to occur.
E)None of these choices are likely to occur.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
80
An endergonic SN2 reaction will have a have a higher energy of activation ( G‡)than an exergonic SN2 reaction.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck



