Deck 5: Chemical Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/88
Play
Full screen (f)
Deck 5: Chemical Reactions
1
Which of the following equations (not balanced)represents an oxidation-reduction process?
A)AgNO3 + NaCl AgCl + NaNO3
B)C + O2 CO2
C)C2H4 + O2 CO2 + H2O
D)more than one response is correct
A)AgNO3 + NaCl AgCl + NaNO3
B)C + O2 CO2
C)C2H4 + O2 CO2 + H2O
D)more than one response is correct
more than one response is correct
2
Single replacement reactions are always
A)redox reactions.
B)nonredox reactions.
C)combination reactions.
D)decomposition reactions.
A)redox reactions.
B)nonredox reactions.
C)combination reactions.
D)decomposition reactions.
redox reactions.
3
Which of the following reactions is correctly balanced?
A)N2 + H 2NH3
B)2H2O + C CO + 2H2
C)Zn + 2HCl H2 + ZnCl2
D)CO + O2 CO2
A)N2 + H 2NH3
B)2H2O + C CO + 2H2
C)Zn + 2HCl H2 + ZnCl2
D)CO + O2 CO2
Zn + 2HCl H2 + ZnCl2
4
Which set of coefficients balances the equation? ___CH4 ___C3H8 +___ H2
A)3,1,1
B)3,2,1
C)3,1,2
D)6,2,2
A)3,1,1
B)3,2,1
C)3,1,2
D)6,2,2

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
5
In which of the following does an element have an oxidation number of +4?
A)TiO2
B)HBr
C)SO3
D)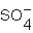
A)TiO2
B)HBr
C)SO3
D)
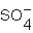

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is an oxidizing agent?
A)O2
B)H2
C)H2O
D)HCl
A)O2
B)H2
C)H2O
D)HCl

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
7
When the equation below is properly balanced,what coefficient is in front of KCl? KClO3 KCl + O2
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
8
A REDOX reaction can also be a(n)
A)combination reaction.
B)single replacement reaction.
C)decomposition reaction.
D)More than one response is correct.
A)combination reaction.
B)single replacement reaction.
C)decomposition reaction.
D)More than one response is correct.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following statements is not true about the reaction? 2 CH4 + 3 O2 2 CO2 + 4 H2O
A)Water is a reactant.
B)Methane and carbon dioxide are products.
C)Methane is a product.
D)Water and carbon dioxide are products.
A)Water is a reactant.
B)Methane and carbon dioxide are products.
C)Methane is a product.
D)Water and carbon dioxide are products.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
10
What is the oxidation number of Cl in HClO3?
A)+2
B)+3
C)+4
D)+5
A)+2
B)+3
C)+4
D)+5

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
11
Which species would be considered a spectator ion based on the following equation? Ba2+ + 2NO3 - + 2Na+ + SO42+ BaSO4(s) + 2NO3 - + 2Na+
A)Ba2+
B)SO42+
C)BaSO4
D)2NO3 -
A)Ba2+
B)SO42+
C)BaSO4
D)2NO3 -

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
12
Propane,C3H8,burns to produce carbon dioxide and water by the equation below.What is the coefficient in front of the carbon dioxide in the balanced equation? C3H8 + O2 CO2 + H2O
A)1
B)2
C)3
D)6
A)1
B)2
C)3
D)6

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
13
What is the oxidizing agent in the following reaction? 4HCl + MnO2 Cl2 + 2H2O + MnCl2
A)HCl
B)Cl2
C)MnO2
D)MnCl2
A)HCl
B)Cl2
C)MnO2
D)MnCl2

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
14
What substance is reduced in the following reaction? 4HCl + MnO2 Cl2 + 2H2O + MnCl2
A)Cl in HCl
B)Mn in MnO2
C)H in HCl
D)O in MnO2
A)Cl in HCl
B)Mn in MnO2
C)H in HCl
D)O in MnO2

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
15
What is the oxidation number of Br in 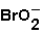 ?
?
A)"-1"
B)"+1"
C)"+2"
D)"+3"
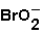 ?
?A)"-1"
B)"+1"
C)"+2"
D)"+3"

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
16
Consider the reactants and propose the right side of the equation. Mg + H2SO4 ______
A)no reaction
B)MgHSO4
C)MgSO4 + H2
D)MgH2 + SO4
A)no reaction
B)MgHSO4
C)MgSO4 + H2
D)MgH2 + SO4

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
17
What is the oxidation number of Mn in KMnO4?
A)+9
B)+5
C)+7
D)+4
A)+9
B)+5
C)+7
D)+4

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
18
Consider the reactants in the partial equation given.Which choice is a product? H3PO4 + Sr(OH)2 _______
A)no reaction
B)SrH3PO5
C)SrH5PO6
D)Sr3(PO4)2
A)no reaction
B)SrH3PO5
C)SrH5PO6
D)Sr3(PO4)2

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following contains the metal with the highest oxidation number?
A)CaCl2
B)NaCl
C)FeCl3
D)CuCl2
A)CaCl2
B)NaCl
C)FeCl3
D)CuCl2

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
20
What substance is oxidized in the following reaction? 4HCl + MnO2 Cl2 + 2H2O + MnCl2
A)Cl in HCl
B)Mn in MnO2
C)H in HCl
D)O in MnO2
A)Cl in HCl
B)Mn in MnO2
C)H in HCl
D)O in MnO2

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
21
Which ions in the following reaction would be classified as spectator ions? Zn + 2H+ + 2Cl Zn2+ + 2Cl - + H2
A)Zn2+
B)H+
C)Cl -
D)More than one response is correct.
A)Zn2+
B)H+
C)Cl -
D)More than one response is correct.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following is a decomposition reaction?
A)SO2 + O2 2SO3
B)2C2H2 + 5O2 4CO2 + 2H2O
C)2H2O2 2H2O + O2
D)AgNO3 + NaCl AgCl + NaNO3
A)SO2 + O2 2SO3
B)2C2H2 + 5O2 4CO2 + 2H2O
C)2H2O2 2H2O + O2
D)AgNO3 + NaCl AgCl + NaNO3

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following is a single replacement reaction?
A)CuO + H2 Cu + H2O
B)HBr + KOH H2O + KBr
C)SO2 + H2O H2SO3
D)2HI I2 + H2
A)CuO + H2 Cu + H2O
B)HBr + KOH H2O + KBr
C)SO2 + H2O H2SO3
D)2HI I2 + H2

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
24
Acetylene,C2H2,burns according to the following reaction: C2H2+5O2 4CO2+2H2O.Suppose 1.20 g of C2H2 is mixed with 3.50 g of O2 in a closed,steel container,and the mixture is ignited.What substances will be found in the mixture left when the burning is complete?
A)CO2 and H2
B)O2,CO2,and H2O
C)C2H2,CO2,and H2O
D)O2,C2H2,CO2,and H2O
A)CO2 and H2
B)O2,CO2,and H2O
C)C2H2,CO2,and H2O
D)O2,C2H2,CO2,and H2O

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
25
How many grams of N2 are required to completely react with 3.03 grams of H2 for the following balanced chemical equation? N2 + 3H2 2NH3
A)1.00
B)6.00
C)14.0
D)28.0
A)1.00
B)6.00
C)14.0
D)28.0

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
26
In the reaction 2CH4 + O2 2CO + 4H2,how many molecules of CO will be produced by the complete reaction of 1.60 grams of CH4?
A)12.0 *1023
B)3.01 * 1022
C)6.02 * 1022
D)2.00
A)12.0 *1023
B)3.01 * 1022
C)6.02 * 1022
D)2.00

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
27
The energy involved in chemical reactions
A)always appears as heat.
B)always appears as light.
C)always appears as electricity.
D)can take many forms.
A)always appears as heat.
B)always appears as light.
C)always appears as electricity.
D)can take many forms.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
28
Which ions will appear in the total ionic equation for the following reaction? 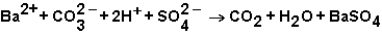
A)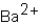 and
and 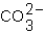
B)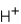 and
and 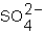
C)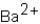 ,
, 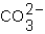 ,and H+
,and H+
D)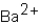 ,
, 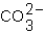 ,
, 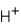 ,and
,and 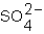
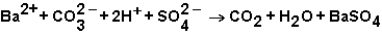
A)
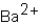 and
and 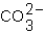
B)
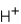 and
and 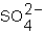
C)
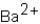 ,
, 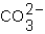 ,and H+
,and H+D)
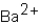 ,
, 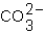 ,
, 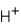 ,and
,and 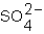

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
29
If 5 grams of CO and 5 grams of O2 are combined according to the reaction,which is the limiting reagent? 2CO + O2 2CO2
A)CO2
B)O2
C)CO
D)CO and O2
A)CO2
B)O2
C)CO
D)CO and O2

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
30
For the reaction of HCl and NaOH,which of the following type of chemicals will appear in the net equation?
A)weak acid
B)weak base
C)salt
D)water
A)weak acid
B)weak base
C)salt
D)water

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following is a double replacement reaction?
A)CuO + H2 Cu + H2O
B)HBr + KOH H2O + KBr
C)SO2 + H2O H2SO3
D)2HI I2 + H2
A)CuO + H2 Cu + H2O
B)HBr + KOH H2O + KBr
C)SO2 + H2O H2SO3
D)2HI I2 + H2

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
32
The Haber Process is a method for the production of ammonia,an important chemical intermediate.The reaction for this process is N2 + 3H2 2NH3
According to the mole interpretation,how many grams of NH3 could be produced by the complete reaction of 2.80 grams of N2 and excess H2?
A)34.0
B)17.0
C)3.10
D)3.40
According to the mole interpretation,how many grams of NH3 could be produced by the complete reaction of 2.80 grams of N2 and excess H2?
A)34.0
B)17.0
C)3.10
D)3.40

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
33
In the reaction 2H2O 2H2 + O2,2.0 mol water will produce how many grams of O2?
A)16
B)32
C)36
D)64
A)16
B)32
C)36
D)64

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
34
Oxygen can be prepared in the lab by heating potassium chlorate in the presence of a catalyst.The reaction is 2KClO3 2KCl + 3O2.How many moles of O2 could be obtained from one mole of KClO3?
A)1.5
B)3.0
C)1.0
D)2.0
A)1.5
B)3.0
C)1.0
D)2.0

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following is a combination reaction?
A)SO2 + O2 2SO3
B)2C2H2 + 5O2 4CO2 + 2H2O
C)2H2O2 2H2O + O2
D)AgNO3 + NaCl AgCl + NaNO3
A)SO2 + O2 2SO3
B)2C2H2 + 5O2 4CO2 + 2H2O
C)2H2O2 2H2O + O2
D)AgNO3 + NaCl AgCl + NaNO3

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
36
What is the reducing agent in the following reaction? 4HCl + MnO2 Cl2 + 2H2O + MnCl2
A)HCl
B)Cl2
C)MnO2
D)MnCl2
A)HCl
B)Cl2
C)MnO2
D)MnCl2

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
37
The Haber Process is a method for the production of ammonia,an important chemical intermediate.The reaction for this process is: N2 + 3H2 2NH3.When the equation is correctly interpreted in terms of moles,how many moles of H2 will react with one mole of N2?
A)1
B)2
C)3
D)6
A)1
B)2
C)3
D)6

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
38
In the equation for an endothermic reaction,the word "heat" appears
A)on the right side.
B)on the left side.
C)on both sides.
D)on neither side.
A)on the right side.
B)on the left side.
C)on both sides.
D)on neither side.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
39
Barium sulfate is often ingested to aid in the diagnosis of GI tract disorders.The equation for its production is Ba2+ + SO BaSO4(s).What type of equation is this?
A)a total ionic equation
B)a full equation
C)an oxidation-reduction equation
D)a net ionic equation
A)a total ionic equation
B)a full equation
C)an oxidation-reduction equation
D)a net ionic equation

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
40
Given the equation 2CO + O2 2CO2,how many grams of CO2 form if 34.0 grams of CO are combined with excess oxygen?
A)53.4
B)21.6
C)145
D)107
A)53.4
B)21.6
C)145
D)107

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
41
A percentage yield less than 100% is often obtained in a reaction.What is(are)the reason for obtaining less than theoretically predicted?
A)Side reactions occurred
B)poor laboratory technique
C)reaction did not go to completion
D)all of the choices
A)Side reactions occurred
B)poor laboratory technique
C)reaction did not go to completion
D)all of the choices

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
42
A container is filled with 4.0 g H2 and 5.0 g O2.The mixture is ignited to produce water according to the following equation.How much water is produced? 2H2 + O2 2H2O
A)4.0 g
B)5.0 g
C)5.6 g
D)9.0 g
A)4.0 g
B)5.0 g
C)5.6 g
D)9.0 g

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following conversion factors would allow one to calculate the amount of PbI2 produced from a given amount of NaI in the following unbalanced reaction? Pb(NO3)2 + Nal Pbl2 + NaNO3
A)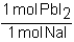
B)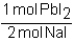
C)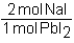
D)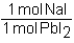
A)
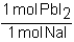
B)
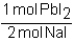
C)
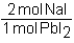
D)
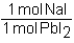

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
44
Suppose 2.43 g of magnesium is reacted with 3.20 g of oxygen: 2Mg + O2 2MgO.How many grams of MgO will be produced?
A)5.63
B)1.27
C)8.06
D)4.03
A)5.63
B)1.27
C)8.06
D)4.03

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
45
Tincture of iodine is an antiseptic.Which of the following is another antiseptic?
A)5% sodium hypochlorite
B)3% hydrogen peroxide
C)ozone
D)All are antiseptics
A)5% sodium hypochlorite
B)3% hydrogen peroxide
C)ozone
D)All are antiseptics

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
46
According to the law of conservation of matter,which of the following may NEVER occur as a result of a chemical reaction?
A)creation of new chemical species.
B)reaction of starting materials.
C)rearranged to form new substances.
D)reduction in total mass.
A)creation of new chemical species.
B)reaction of starting materials.
C)rearranged to form new substances.
D)reduction in total mass.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
47
The modern definition of oxidation is a process
A)involving a reaction with oxygen.
B)in which hydrogen is gained.
C)in which electrons are gained.
D)none of the choices.
A)involving a reaction with oxygen.
B)in which hydrogen is gained.
C)in which electrons are gained.
D)none of the choices.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
48
A percentage yield greater than 100% has been reported in a scientific paper you are assigned to read.What would be a reasonable explanation for this?
A)A lack of training of the professor who published the study.
B)The product was measured more than once.
C)Small measurement errors could have caused this to occur.
D)It must be a typographical error since yields can't exceed 100%.
A)A lack of training of the professor who published the study.
B)The product was measured more than once.
C)Small measurement errors could have caused this to occur.
D)It must be a typographical error since yields can't exceed 100%.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
49
Consider the following chemical equation.Which substance is oxidized? BaCl2 (aq)+ H2SO4 (aq) BaSO4 (s)+ 2HCl (aq)
A)Ba2+
B)Cl- and H+
C)SO42-
D)none of them
A)Ba2+
B)Cl- and H+
C)SO42-
D)none of them

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
50
Which is not a meaning of oxidation?
A)to gain electrons
B)to lose hydrogen
C)to combine with oxygen
D)to lose electrons
A)to gain electrons
B)to lose hydrogen
C)to combine with oxygen
D)to lose electrons

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
51
Automotive car batteries rely on the reaction shown below.Identify the spectator ion(s). PbO2(s)+ 4 H+(aq)+ 2 SO42-(aq)+ Pb(s)- 2 PbSO4(s)+ 2 H2O(l)
A)SO42-
B)H+
C)H+ and SO42-
D)There are no spectator ions.
A)SO42-
B)H+
C)H+ and SO42-
D)There are no spectator ions.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
52
When nitrogen (N2)reacts with sodium (Na),sodium nitride (Na3N)is formed.The balanced equation for the reaction would be:
A)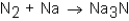
B)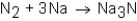
C)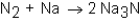
D)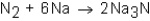
A)
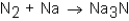
B)
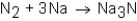
C)
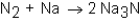
D)
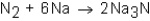

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following phrases can be used to describe the process of reduction?
A)to gain oxygen
B)to combine with hydrogen
C)to gain electrons
D)to decrease in oxidation number
A)to gain oxygen
B)to combine with hydrogen
C)to gain electrons
D)to decrease in oxidation number

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
54
The reaction 2KBr + F2 2KF + Br2 is an example of a _____ reaction.
A)spectator
B)metaphysical
C)single replacement
D)double replacement
A)spectator
B)metaphysical
C)single replacement
D)double replacement

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following types of chemical reaction classes always involve oxidation?
A)single replacement
B)double replacement
C)combination
D)decomposition
A)single replacement
B)double replacement
C)combination
D)decomposition

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
56
When the following equation is balanced,how many oxygen atoms are present on the reactant side of the equation? C2H6 + O2 CO2 + H2O
A)14
B)7
C)3
D)20
A)14
B)7
C)3
D)20

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
57
What is the assigned oxidation number for molybdenum in aluminum molybdate,Al2(MoO4)3?
A)+2
B)+3
C)+6
D)+7
A)+2
B)+3
C)+6
D)+7

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
58
What are coefficients needed to balance the following reaction? 2 CH4 + ____ O2 ____ CO2 + ____ H2O
A)8,2,4
B)4,1,2
C)4,2,4
D)none of these
A)8,2,4
B)4,1,2
C)4,2,4
D)none of these

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
59
The reaction below would be considered _____ . CaCO3 + heat CaO + CO2
A)an endothermic combination.
B)an exothermic combination.
C)an endothermic decomposition.
D)an exothermic decomposition.
A)an endothermic combination.
B)an exothermic combination.
C)an endothermic decomposition.
D)an exothermic decomposition.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
60
The following would represent what type of chemical reaction? 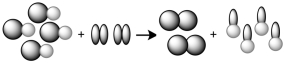
A)decomposition.
B)combination.
C)single replacement.
D)double replacement.

A)decomposition.
B)combination.
C)single replacement.
D)double replacement.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
61
Air bags serve an important role in providing driver and front-seat passenger protection in the event of an automobile accident.They rely on several chemical reactions.Which of the following chemicals is responsible for N2 production?
A)sodium azide
B)potassium nitrate
C)silicon dioxide
D)more than one answer is correct.
A)sodium azide
B)potassium nitrate
C)silicon dioxide
D)more than one answer is correct.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
62
In the reaction Zn + Cu(NO3)2 Cu + Zn(NO3),the Zn is reduced.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
63
In an exothermic reaction,heat is liberated to the surroundings.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
64
Most reactions give a percent yield of almost 100%.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
65
Water is a product when any inorganic acid reacts with any inorganic base.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
66
The reaction Mg + 3N2 Mg3N2 is correctly balanced as written.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
67
Products are always on the right side of a chemical equation.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
68
The reactions NaOH + HCl H2O + NaCl and Ca(OH)2 + 2HBr 2H2O + CaBr2 have the same net ionic equation.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
69
Some redox reactions involve only oxidation without any reduction.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
70
Lycopene,a phytonutrient,has several potential health benefits,including protection against prostate,cervical and pancreatic cancer.Which of the following is a good source of this substance?
A)spinach
B)broccoli
C)strawberries
D)tomatoes
A)spinach
B)broccoli
C)strawberries
D)tomatoes

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
71
All redox reactions are exothermic reactions.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
72
The reducing agent is the substance that is reduced in the reaction.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
73
In ionic compounds,metals have a positive oxidation number.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
74
The oxidation number of iodine in HIO4 is +6.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
75
The net ionic equation for the reaction H2SO4 + NaOH Na2SO4 + H2O is
H+ + OH H2O.
H+ + OH H2O.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
76
The reaction Ca + Cl2 CaCl2 is a redox reaction.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
77
The reaction AgNO3 + HCl AgCl + HNO3 is a redox reaction.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
78
A net ionic equation may include one or more spectator ions.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
79
Redox reactions may also be a decomposition reaction.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
80
In the reaction CuCl2 + Fe Cu + FeCl2 the CuCl2 is the oxidizing agent.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck



