Deck 13: Chemical Kinetics-Clearing the Air
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/213
Play
Full screen (f)
Deck 13: Chemical Kinetics-Clearing the Air
1
Indicate which of the following compounds is a component of photochemical smog.
A)H2O
B)CO2
C)N2O
D)O3
E)CO
A)H2O
B)CO2
C)N2O
D)O3
E)CO
O3
2
The greatest NO concentration is observed __________
A)in the morning before rush hour.
B)in the morning just after rush hour.
C)in midmorning.
D)at noon.
E)in midafternoon.
A)in the morning before rush hour.
B)in the morning just after rush hour.
C)in midmorning.
D)at noon.
E)in midafternoon.
in the morning just after rush hour.
3
For the reaction 2 A + 3 B 4 C + 5 D,the rate of the reaction in terms of [A] would be written as __________
A)![<strong>For the reaction 2 A + 3 B \to 4 C + 5 D,the rate of the reaction in terms of \Delta [A] would be written as __________</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35cf_2fd7_b44f_5dc67a2f89d8_TB6561_11_TB6561_11.jpg)
B)![<strong>For the reaction 2 A + 3 B \to 4 C + 5 D,the rate of the reaction in terms of \Delta [A] would be written as __________</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35cf_56e8_b44f_cb73b9f5050a_TB6561_11_TB6561_11.jpg)
C)![<strong>For the reaction 2 A + 3 B \to 4 C + 5 D,the rate of the reaction in terms of \Delta [A] would be written as __________</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35cf_7df9_b44f_4152387e4b2d_TB6561_11_TB6561_11.jpg)
D)![<strong>For the reaction 2 A + 3 B \to 4 C + 5 D,the rate of the reaction in terms of \Delta [A] would be written as __________</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35cf_a50a_b44f_8dd581cc33b6_TB6561_11_TB6561_11.jpg)
E)![<strong>For the reaction 2 A + 3 B \to 4 C + 5 D,the rate of the reaction in terms of \Delta [A] would be written as __________</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35cf_a50b_b44f_f1f47aa9ba21_TB6561_11_TB6561_11.jpg)
A)
![<strong>For the reaction 2 A + 3 B \to 4 C + 5 D,the rate of the reaction in terms of \Delta [A] would be written as __________</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35cf_2fd7_b44f_5dc67a2f89d8_TB6561_11_TB6561_11.jpg)
B)
![<strong>For the reaction 2 A + 3 B \to 4 C + 5 D,the rate of the reaction in terms of \Delta [A] would be written as __________</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35cf_56e8_b44f_cb73b9f5050a_TB6561_11_TB6561_11.jpg)
C)
![<strong>For the reaction 2 A + 3 B \to 4 C + 5 D,the rate of the reaction in terms of \Delta [A] would be written as __________</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35cf_7df9_b44f_4152387e4b2d_TB6561_11_TB6561_11.jpg)
D)
![<strong>For the reaction 2 A + 3 B \to 4 C + 5 D,the rate of the reaction in terms of \Delta [A] would be written as __________</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35cf_a50a_b44f_8dd581cc33b6_TB6561_11_TB6561_11.jpg)
E)
![<strong>For the reaction 2 A + 3 B \to 4 C + 5 D,the rate of the reaction in terms of \Delta [A] would be written as __________</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35cf_a50b_b44f_f1f47aa9ba21_TB6561_11_TB6561_11.jpg)
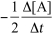
4
Which of the following statements regarding catalytic converters is NOT correct?
A)A catalytic converter targets compounds such as hydrocarbons,carbon monoxide,and nitrogen oxide compounds.
B)Reactions that reduce the amounts of hazardous by-products of the combustion reactions in automobiles occur very rapidly in the catalytic converter.
C)CO is usually oxidized to CO2 in one stage of the catalytic converter.
D)Nitrogen oxides are usually oxidized to N2 and O2 in the catalytic converter.
E)Hydrocarbons are usually oxidized to CO2 and H2O in the catalytic converter.
A)A catalytic converter targets compounds such as hydrocarbons,carbon monoxide,and nitrogen oxide compounds.
B)Reactions that reduce the amounts of hazardous by-products of the combustion reactions in automobiles occur very rapidly in the catalytic converter.
C)CO is usually oxidized to CO2 in one stage of the catalytic converter.
D)Nitrogen oxides are usually oxidized to N2 and O2 in the catalytic converter.
E)Hydrocarbons are usually oxidized to CO2 and H2O in the catalytic converter.

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
5
Indicate which of the following compounds is NOT a component of photochemical smog.
A)O3
B)H2O
C)NO2
D)NO
E)organic molecules
A)O3
B)H2O
C)NO2
D)NO
E)organic molecules

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
6
At what time of day are ozone concentrations highest during a photochemical smog event?
A)in the morning before rush hour
B)in the morning just after rush hour
C)midmorning
D)noon
E)midafternoon
A)in the morning before rush hour
B)in the morning just after rush hour
C)midmorning
D)noon
E)midafternoon

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
7
For the reaction 2 A + 3 B 4 C + 5 D,the rate of the reaction in terms of [B] would be written as __________
A)![<strong>For the reaction 2 A + 3 B \to 4 C + 5 D,the rate of the reaction in terms of \Delta [B] would be written as __________</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35cf_f32c_b44f_3346ca5f4ac9_TB6561_11_TB6561_11.jpg)
B)![<strong>For the reaction 2 A + 3 B \to 4 C + 5 D,the rate of the reaction in terms of \Delta [B] would be written as __________</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35d0_1a3d_b44f_f7c7efed2472_TB6561_11_TB6561_11.jpg)
C)![<strong>For the reaction 2 A + 3 B \to 4 C + 5 D,the rate of the reaction in terms of \Delta [B] would be written as __________</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35d0_414e_b44f_7bde1f71e868_TB6561_11_TB6561_11.jpg)
D)![<strong>For the reaction 2 A + 3 B \to 4 C + 5 D,the rate of the reaction in terms of \Delta [B] would be written as __________</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35d0_685f_b44f_27d7fc314670_TB6561_11_TB6561_11.jpg)
E)![<strong>For the reaction 2 A + 3 B \to 4 C + 5 D,the rate of the reaction in terms of \Delta [B] would be written as __________</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35d0_8f70_b44f_ad33970e8730_TB6561_11_TB6561_11.jpg)
A)
![<strong>For the reaction 2 A + 3 B \to 4 C + 5 D,the rate of the reaction in terms of \Delta [B] would be written as __________</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35cf_f32c_b44f_3346ca5f4ac9_TB6561_11_TB6561_11.jpg)
B)
![<strong>For the reaction 2 A + 3 B \to 4 C + 5 D,the rate of the reaction in terms of \Delta [B] would be written as __________</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35d0_1a3d_b44f_f7c7efed2472_TB6561_11_TB6561_11.jpg)
C)
![<strong>For the reaction 2 A + 3 B \to 4 C + 5 D,the rate of the reaction in terms of \Delta [B] would be written as __________</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35d0_414e_b44f_7bde1f71e868_TB6561_11_TB6561_11.jpg)
D)
![<strong>For the reaction 2 A + 3 B \to 4 C + 5 D,the rate of the reaction in terms of \Delta [B] would be written as __________</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35d0_685f_b44f_27d7fc314670_TB6561_11_TB6561_11.jpg)
E)
![<strong>For the reaction 2 A + 3 B \to 4 C + 5 D,the rate of the reaction in terms of \Delta [B] would be written as __________</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35d0_8f70_b44f_ad33970e8730_TB6561_11_TB6561_11.jpg)

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
8
NO2 contributes to the "brown haze" associated with photochemical smog events.At what time of day is NO2 concentration highest?
A)in the morning before rush hour
B)in the morning just after rush hour
C)midmorning
D)noon
E)midafternoon
A)in the morning before rush hour
B)in the morning just after rush hour
C)midmorning
D)noon
E)midafternoon

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
9
For the reaction CH4(g)+ 4 NO(g) 2 N2(g)+ CO2(g)+ 2 H2O(g),the rate of the reaction in terms of the change in nitrogen monoxide concentration would be written as __________
A)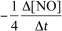
B)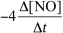
C)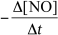
D)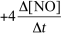
E)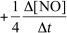
A)
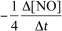
B)
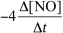
C)
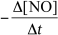
D)
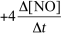
E)
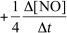

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
10
If the rate of formation of ammonia is 0.345 M/s,what is the rate of disappearance of N2? N2(g)+ 3 H2(g) 2 NH3(g)
A)0.173 M/s
B)0.345 M/s
C)0.690 M/s
D)245 M/s
E)0.518 M/s
A)0.173 M/s
B)0.345 M/s
C)0.690 M/s
D)245 M/s
E)0.518 M/s

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
11
In the combustion of methane,CH4(g)+ 2 O2(g) CO2(g)+ 2 H2O(g),which reactant has the greatest rate of disappearance?
A)CH4
B)O2
C)CO2
D)H2O
E)CH4 and O2 have the same rate of disappearance.
A)CH4
B)O2
C)CO2
D)H2O
E)CH4 and O2 have the same rate of disappearance.

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
12
The order in which ozone,nitrogen monoxide,and nitrogen dioxide build up in the atmosphere over the course of the day is __________ then __________ then __________
A)NO; NO2; O3.
B)NO; O3; NO2.
C)NO2; NO; O3.
D)NO2; O3; NO.
E)O3; NO; NO2.
A)NO; NO2; O3.
B)NO; O3; NO2.
C)NO2; NO; O3.
D)NO2; O3; NO.
E)O3; NO; NO2.

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
13
HI dissociates to form I2 and H2: 2 HI(g) H2(g)+ I2(g). If the concentration of HI changes at a rate of -0.45 M/s,what is the rate of appearance of I2(g)?
A)0.90 M/s
B)0.45 M/s
C)0.23 M/s
D)1.00 M/s
E)0.13 M/s
A)0.90 M/s
B)0.45 M/s
C)0.23 M/s
D)1.00 M/s
E)0.13 M/s

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is NOT an important reaction in the production of photochemical smog?
A)H2O2(g)+ O2(g) O3(g)+ H2O(g)
B)N2(g)+ O2(g) 2 NO(g)
C)O3(g)+ NO(g) O2(g)+ NO2(g)
D)NO2(g) NO(g)+ O(g)
E)N2(g)+ 3 O2(g)+ OH(g)+ CH3CHO(g) CH3(O)O2NO2(g)+ H2O(g)+ NO2(g)
A)H2O2(g)+ O2(g) O3(g)+ H2O(g)
B)N2(g)+ O2(g) 2 NO(g)
C)O3(g)+ NO(g) O2(g)+ NO2(g)
D)NO2(g) NO(g)+ O(g)
E)N2(g)+ 3 O2(g)+ OH(g)+ CH3CHO(g) CH3(O)O2NO2(g)+ H2O(g)+ NO2(g)

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
15
For the reaction CH4(g)+ 4 NO(g) 2 N2(g)+ CO2(g)+ 2 H2O(g),the rate of the reaction in terms of the change in nitrogen concentration would be written as __________
A)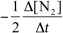
B)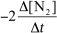
C)
D)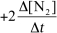
E)
A)
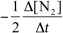
B)
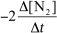
C)

D)
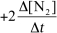
E)


Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
16
Smog created by the interaction of sunlight with nitrogen oxides and volatile organic compounds is termed __________ smog.
A)photolytic
B)photochemical
C)photophysical
D)acid
E)sulfurous
A)photolytic
B)photochemical
C)photophysical
D)acid
E)sulfurous

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following is NOT important as a contributing factor to photochemical smog?
A)stagnant air
B)sunlight
C)a lot of traffic
D)irrigation
E)cars without catalytic converters
A)stagnant air
B)sunlight
C)a lot of traffic
D)irrigation
E)cars without catalytic converters

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
18
What is the device in an automobile that decreases the amount of NO and partially oxidized hydrocarbons in exhaust gases?
A)a catalytic intermediate
B)a photochemical inhibitor
C)a homogeneous catalyst
D)a nitrogen monoxide reducer
E)a catalytic converter
A)a catalytic intermediate
B)a photochemical inhibitor
C)a homogeneous catalyst
D)a nitrogen monoxide reducer
E)a catalytic converter

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
19
For the reaction 2 A + 3 B 4 C + 5 D,the rate of the reaction in terms of [C] would be written as __________.
A)![<strong>For the reaction 2 A + 3 B \to 4 C + 5 D,the rate of the reaction in terms of \Delta [C] would be written as __________.</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35d0_b681_b44f_5117ddc6c910_TB6561_11_TB6561_11.jpg)
B)![<strong>For the reaction 2 A + 3 B \to 4 C + 5 D,the rate of the reaction in terms of \Delta [C] would be written as __________.</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35d0_dd92_b44f_e5e4e9767495_TB6561_11_TB6561_11.jpg)
C)![<strong>For the reaction 2 A + 3 B \to 4 C + 5 D,the rate of the reaction in terms of \Delta [C] would be written as __________.</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35d1_04a3_b44f_3d1d178fa1dd_TB6561_11_TB6561_11.jpg)
D)![<strong>For the reaction 2 A + 3 B \to 4 C + 5 D,the rate of the reaction in terms of \Delta [C] would be written as __________.</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35d1_04a4_b44f_a1fc323452d2_TB6561_11_TB6561_11.jpg)
E)![<strong>For the reaction 2 A + 3 B \to 4 C + 5 D,the rate of the reaction in terms of \Delta [C] would be written as __________.</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35d1_2bb5_b44f_679ed2384e7d_TB6561_11_TB6561_11.jpg)
A)
![<strong>For the reaction 2 A + 3 B \to 4 C + 5 D,the rate of the reaction in terms of \Delta [C] would be written as __________.</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35d0_b681_b44f_5117ddc6c910_TB6561_11_TB6561_11.jpg)
B)
![<strong>For the reaction 2 A + 3 B \to 4 C + 5 D,the rate of the reaction in terms of \Delta [C] would be written as __________.</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35d0_dd92_b44f_e5e4e9767495_TB6561_11_TB6561_11.jpg)
C)
![<strong>For the reaction 2 A + 3 B \to 4 C + 5 D,the rate of the reaction in terms of \Delta [C] would be written as __________.</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35d1_04a3_b44f_3d1d178fa1dd_TB6561_11_TB6561_11.jpg)
D)
![<strong>For the reaction 2 A + 3 B \to 4 C + 5 D,the rate of the reaction in terms of \Delta [C] would be written as __________.</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35d1_04a4_b44f_a1fc323452d2_TB6561_11_TB6561_11.jpg)
E)
![<strong>For the reaction 2 A + 3 B \to 4 C + 5 D,the rate of the reaction in terms of \Delta [C] would be written as __________.</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35d1_2bb5_b44f_679ed2384e7d_TB6561_11_TB6561_11.jpg)

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
20
Large cities often issue ozone advisories.At what time of year would an advisory be likely to occur most often?
A)winter
B)spring
C)summer
D)fall
E)No season should have more advisories than another.
A)winter
B)spring
C)summer
D)fall
E)No season should have more advisories than another.

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
21
For the reaction CH4(g)+ 4 NO(g) 2 N2(g)+ CO2(g)+ 2 H2O(g),the rate of the production of water is __________ that of the rate of consumption of NO.
A)twice
B)half
C)four times
D)one-quarter
E)equal to
A)twice
B)half
C)four times
D)one-quarter
E)equal to

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
22
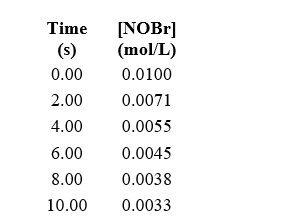
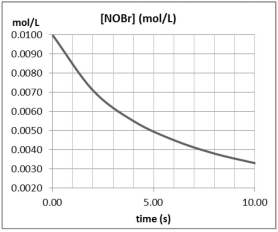
What is the average reaction rate for 2 NOBr(g) Br2(g)+ 2 NO(g)over the first 10 seconds?
A)1.3 10-3 M/s
B)3.3 10-3 M/s
C)6.7 10-3 M/s
D)6.7 10-4 M/s
E)3.4 10-4 M/s


A)1.3 10-3 M/s
B)3.3 10-3 M/s
C)6.7 10-3 M/s
D)6.7 10-4 M/s
E)3.4 10-4 M/s

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
23
Ammonia gas (NH3)is produced from hydrogen and nitrogen gas according to the following reaction: 3 H2(g)+ N2(g) 2 NH3(g)
If the rate of production of ammonia is R(NH3),what is the rate of loss of hydrogen and nitrogen gas,respectively?
A)-R(H2)= 2/3 R(NH3); -R(N2)= 1/2 R(NH3)
B)-R(H2)= 3/2 R(NH3); -R(N2)= 2 R(NH3)
C)-R(H2)= 3/2 R(NH3); -R(N2)= 1/2 R(NH3)
D)-R(H2)= 2/3 R(NH3); -R(N2)= 2 R(NH3)
E)-R(H2)= R(NH3); -R(N2)= R(NH3)
If the rate of production of ammonia is R(NH3),what is the rate of loss of hydrogen and nitrogen gas,respectively?
A)-R(H2)= 2/3 R(NH3); -R(N2)= 1/2 R(NH3)
B)-R(H2)= 3/2 R(NH3); -R(N2)= 2 R(NH3)
C)-R(H2)= 3/2 R(NH3); -R(N2)= 1/2 R(NH3)
D)-R(H2)= 2/3 R(NH3); -R(N2)= 2 R(NH3)
E)-R(H2)= R(NH3); -R(N2)= R(NH3)

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following does the initial rate of a chemical reaction depend on?
I.Concentration of reactants
II.Concentration of products
III.Temperature
A)I
B)III
C)II
D)Both I and III
E)I,II,and III
I.Concentration of reactants
II.Concentration of products
III.Temperature
A)I
B)III
C)II
D)Both I and III
E)I,II,and III

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
25
If the rate of formation of ammonia is 0.345 M/s,what is the rate of disappearance of N2?
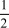 N2(g)+
N2(g)+ 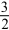 H2(g) NH3(g)
H2(g) NH3(g)
A)0.173 M/s
B)0.345 M/s
C)0.690 M/s
D)245 M/s
E)0.518 M/s
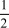 N2(g)+
N2(g)+ 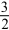 H2(g) NH3(g)
H2(g) NH3(g)A)0.173 M/s
B)0.345 M/s
C)0.690 M/s
D)245 M/s
E)0.518 M/s

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
26
The rate of disappearance of HI in the reaction 2 HI(g) I2(g)+ H2(g)is shown in the following figure.Estimate the instantaneous rate of this reaction at t = 5 s. 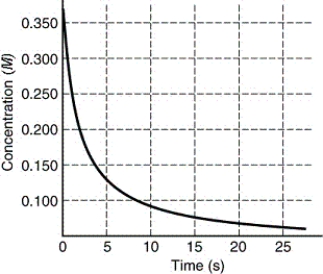
A)0.2 M/s
B)0.01 M/s
C)0.02 M/s
D)0.1 M/s
E)0.04 M/s

A)0.2 M/s
B)0.01 M/s
C)0.02 M/s
D)0.1 M/s
E)0.04 M/s

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following statements regarding the average rate of a reaction is NOT true?
A)The change in concentration of reactant or product is not always linear with time.
B)The average reaction rate usually decreases as the reaction proceeds.
C)The average reaction rate can be determined from the slope of a line drawn between two points on a plot of concentration versus time.
D)The average reaction rate can be determined from the slope of the tangent line drawn at time = 0 on a plot of concentration versus time.
E)The average rate usually depends on the concentrations of reactants.
A)The change in concentration of reactant or product is not always linear with time.
B)The average reaction rate usually decreases as the reaction proceeds.
C)The average reaction rate can be determined from the slope of a line drawn between two points on a plot of concentration versus time.
D)The average reaction rate can be determined from the slope of the tangent line drawn at time = 0 on a plot of concentration versus time.
E)The average rate usually depends on the concentrations of reactants.

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
28
If the average rate of reaction for 2 N2O5(g) 4 NO2(g)+ O2(g)is 1.30 10-5 M/s over five minutes of the reaction,what is the partial pressure of NO2 after the first 5.00 minutes if the reaction takes place in a 10.0 L vessel maintained at 55.0°C?
A)0.420 atm
B)0.105 atm
C)0.156 atm
D)0.0704 atm
E)0.084 atm
A)0.420 atm
B)0.105 atm
C)0.156 atm
D)0.0704 atm
E)0.084 atm

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
29
Approximately how many times faster or slower is the average reaction rate during the first four seconds (t = 0 to t = 4)than the second four seconds (t = 4 to t = 8)for the following reaction? 2 NOBr(g) Br2(g)+ 2 NO(g)
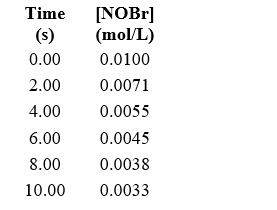
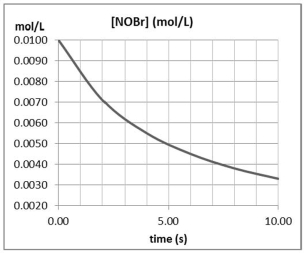
A)about 1.4 times faster
B)about 1.4 times slower
C)about 2.64 times slower
D)about 2.6 times faster
E)The average rate is constant.


A)about 1.4 times faster
B)about 1.4 times slower
C)about 2.64 times slower
D)about 2.6 times faster
E)The average rate is constant.

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
30
For the reaction 4 NH3(g)+ 7 O2(g) 4 NO2(g)+ 6 H2(g),if H2 is produced at a rate of 3.5 10-3 M/s, O2 is consumed at a rate of __________
A)2.5 10-2 M/s.
B)2.1 10-2 M/s.
C)4.1 10-3 M/s.
D)3.0 10-3 M/s.
E)5.8 10-4 M/s.
A)2.5 10-2 M/s.
B)2.1 10-2 M/s.
C)4.1 10-3 M/s.
D)3.0 10-3 M/s.
E)5.8 10-4 M/s.

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
31
For the reaction 3 I-(aq)+ H3AsO4(aq)+ 2 H+(aq) I3-(aq)+ H3AsO3(aq)+ 2 H2O(  ),the rate of I- consumption is __________ that of the rate of production of water.
),the rate of I- consumption is __________ that of the rate of production of water.
A)one third
B)three times
C)two thirds
D)one and a half times
E)equal to
 ),the rate of I- consumption is __________ that of the rate of production of water.
),the rate of I- consumption is __________ that of the rate of production of water.A)one third
B)three times
C)two thirds
D)one and a half times
E)equal to

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following statements is true about the instantaneous rate for the chemical reaction A B as the reaction progresses?
A)"- [A]/ t increases while [B]/ t decreases"
B)"- [A]/ t decreases while [B]/ t increases"
C)"both - [A]/ t and [B]/ t decrease"
D)"both - [A]/ t and [B]/ t increase"
E)"The answer will vary depending on the reaction mechanism."
A)"- [A]/ t increases while [B]/ t decreases"
B)"- [A]/ t decreases while [B]/ t increases"
C)"both - [A]/ t and [B]/ t decrease"
D)"both - [A]/ t and [B]/ t increase"
E)"The answer will vary depending on the reaction mechanism."

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
33
If the average rate of reaction for 2 O3(g) 3 O2(g)is 0.250 M/s over the first 5.50 s,how much oxygen will form during this time?
A)1.38 M
B)4.13 M
C)0.69 M
D)0.25 M
E)0.46 M
A)1.38 M
B)4.13 M
C)0.69 M
D)0.25 M
E)0.46 M

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
34
The rate of disappearance of HI in the reaction 2 HI(g) I2(g)+ H2(g)is shown in the following figure.What is the approximate average rate of reaction over the first 15.0 seconds of the reaction? 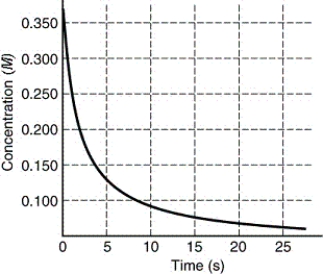
A)0.275 M/s
B)0.138 M/s
C)0.0117 M/s
D)0.00917 M/s
E)0.0183 M/s

A)0.275 M/s
B)0.138 M/s
C)0.0117 M/s
D)0.00917 M/s
E)0.0183 M/s

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
35
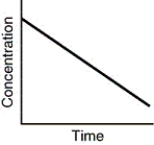
Assuming that each of the following graphs has the same concentration and time axes,which has the greatest initial rate of disappearance of reactant?
A)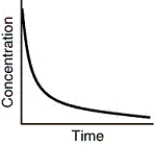
B)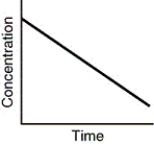
C)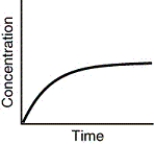
D)all are equivalent at the start of the reaction
A)

B)

C)

D)all are equivalent at the start of the reaction

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following statements regarding the difference between an average rate and an instantaneous rate is NOT correct?
A)The average rate is taken over a larger time period.
B)The instantaneous rate is taken from the slope of the curve at a specific time.
C)They are not different if the time interval chosen is small enough.
D)The instantaneous rate is always faster than the average rate.
E)Both tend to decrease as the reaction proceeds.
A)The average rate is taken over a larger time period.
B)The instantaneous rate is taken from the slope of the curve at a specific time.
C)They are not different if the time interval chosen is small enough.
D)The instantaneous rate is always faster than the average rate.
E)Both tend to decrease as the reaction proceeds.

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following statements regarding the determination of reaction rates is true?
A)Reaction rates can be measured by monitoring how much product is generated or how much reactant is consumed in a given amount of time.
B)Reaction rates can be completely predicted based on the stoichiometry of a reaction.
C)The reaction rate determined at one instant during the course of a reaction is the same as at any other instant.
D)Experimentally determined reaction rates do not depend on factors other than concentration.
E)The average rate of reaction does not vary.
A)Reaction rates can be measured by monitoring how much product is generated or how much reactant is consumed in a given amount of time.
B)Reaction rates can be completely predicted based on the stoichiometry of a reaction.
C)The reaction rate determined at one instant during the course of a reaction is the same as at any other instant.
D)Experimentally determined reaction rates do not depend on factors other than concentration.
E)The average rate of reaction does not vary.

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following is not a possible graph of concentration versus time for a reactant?
A)
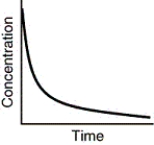
B)
C)
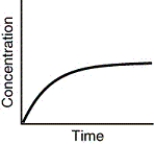
D)
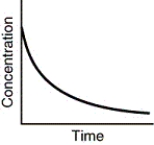
E)all of these are possible
A)

B)

C)

D)

E)all of these are possible

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
39
For the reaction 3 I-(aq)+ H3AsO4(aq)+ 2 H+(aq) I3-(aq)+ H3AsO3(aq)+ 2 H2O(  ),if I- is consumed at a rate of 1.2 10-4 M/s,H2O is produced at a rate of __________.
),if I- is consumed at a rate of 1.2 10-4 M/s,H2O is produced at a rate of __________.
A)8.0 10-5 M/s
B)4.0 10-5 M/s
C)3.6 10-4 M/s
D)2.4 10-4 M/s
E)1.8 10-4 M/s
 ),if I- is consumed at a rate of 1.2 10-4 M/s,H2O is produced at a rate of __________.
),if I- is consumed at a rate of 1.2 10-4 M/s,H2O is produced at a rate of __________.A)8.0 10-5 M/s
B)4.0 10-5 M/s
C)3.6 10-4 M/s
D)2.4 10-4 M/s
E)1.8 10-4 M/s

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
40
The following graph shows the kinetics curves for the reaction of oxygen with hydrogen to form water: O2(g)+ 2 H2(g) 2 H2O(g).Which curve is hydrogen? 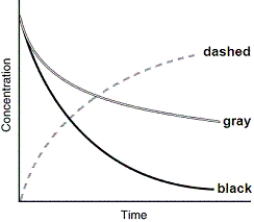
A)the dashed curve
B)the gray curve
C)the black curve
D)either the gray or the black curve
E)Any of these curves could be hydrogen.

A)the dashed curve
B)the gray curve
C)the black curve
D)either the gray or the black curve
E)Any of these curves could be hydrogen.

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
41
The rate of a reaction is found to double when the concentration of one reactant is quadrupled.The order of the reaction with respect to this reactant is __________
A)first.
B)second.
C)one-quarter.
D)one-half.
E)third.
A)first.
B)second.
C)one-quarter.
D)one-half.
E)third.

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
42
For the rate law Rate = k[A]3/2[B],the partial order with respect to A is __________,the partial order with respect to B is __________,and the total order is __________.
A)![<strong>For the rate law Rate = k[A]<sup>3/2</sup>[B],the partial order with respect to A is __________,the partial order with respect to B is __________,and the total order is __________.</strong> A) B) C) D) E)The orders cannot be determined without a chemical reaction.](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35dd_d60d_b44f_0993963550b7_TB6561_11_TB6561_11.jpg)
B)![<strong>For the rate law Rate = k[A]<sup>3/2</sup>[B],the partial order with respect to A is __________,the partial order with respect to B is __________,and the total order is __________.</strong> A) B) C) D) E)The orders cannot be determined without a chemical reaction.](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35dd_fd1e_b44f_c553ef53e8d2_TB6561_11_TB6561_11.jpg)
C)![<strong>For the rate law Rate = k[A]<sup>3/2</sup>[B],the partial order with respect to A is __________,the partial order with respect to B is __________,and the total order is __________.</strong> A) B) C) D) E)The orders cannot be determined without a chemical reaction.](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35de_242f_b44f_e9732c97cf6d_TB6561_11_TB6561_11.jpg)
D)![<strong>For the rate law Rate = k[A]<sup>3/2</sup>[B],the partial order with respect to A is __________,the partial order with respect to B is __________,and the total order is __________.</strong> A) B) C) D) E)The orders cannot be determined without a chemical reaction.](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35de_4b40_b44f_d704142fe8d4_TB6561_11_TB6561_11.jpg)
E)The orders cannot be determined without a chemical reaction.
A)
![<strong>For the rate law Rate = k[A]<sup>3/2</sup>[B],the partial order with respect to A is __________,the partial order with respect to B is __________,and the total order is __________.</strong> A) B) C) D) E)The orders cannot be determined without a chemical reaction.](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35dd_d60d_b44f_0993963550b7_TB6561_11_TB6561_11.jpg)
B)
![<strong>For the rate law Rate = k[A]<sup>3/2</sup>[B],the partial order with respect to A is __________,the partial order with respect to B is __________,and the total order is __________.</strong> A) B) C) D) E)The orders cannot be determined without a chemical reaction.](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35dd_fd1e_b44f_c553ef53e8d2_TB6561_11_TB6561_11.jpg)
C)
![<strong>For the rate law Rate = k[A]<sup>3/2</sup>[B],the partial order with respect to A is __________,the partial order with respect to B is __________,and the total order is __________.</strong> A) B) C) D) E)The orders cannot be determined without a chemical reaction.](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35de_242f_b44f_e9732c97cf6d_TB6561_11_TB6561_11.jpg)
D)
![<strong>For the rate law Rate = k[A]<sup>3/2</sup>[B],the partial order with respect to A is __________,the partial order with respect to B is __________,and the total order is __________.</strong> A) B) C) D) E)The orders cannot be determined without a chemical reaction.](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35de_4b40_b44f_d704142fe8d4_TB6561_11_TB6561_11.jpg)
E)The orders cannot be determined without a chemical reaction.

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
43
For the rate law Rate = k[A][B]1/2,the partial order with respect to A is __________,the partial order with respect to B is __________,and the total order is __________.
A)![<strong>For the rate law Rate = k[A][B]<sup>1/2</sup>,the partial order with respect to A is __________,the partial order with respect to B is __________,and the total order is __________.</strong> A) B) C) D) E)The orders cannot be determined without a chemical reaction.](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35db_6501_b44f_0b06c6070540_TB6561_11_TB6561_11.jpg)
B)![<strong>For the rate law Rate = k[A][B]<sup>1/2</sup>,the partial order with respect to A is __________,the partial order with respect to B is __________,and the total order is __________.</strong> A) B) C) D) E)The orders cannot be determined without a chemical reaction.](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35db_8c12_b44f_d9313ff5a7ae_TB6561_11_TB6561_11.jpg)
C)![<strong>For the rate law Rate = k[A][B]<sup>1/2</sup>,the partial order with respect to A is __________,the partial order with respect to B is __________,and the total order is __________.</strong> A) B) C) D) E)The orders cannot be determined without a chemical reaction.](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35db_8c13_b44f_e51c467dd46e_TB6561_11_TB6561_11.jpg)
D)![<strong>For the rate law Rate = k[A][B]<sup>1/2</sup>,the partial order with respect to A is __________,the partial order with respect to B is __________,and the total order is __________.</strong> A) B) C) D) E)The orders cannot be determined without a chemical reaction.](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35db_b324_b44f_f706158a2a69_TB6561_11_TB6561_11.jpg)
E)The orders cannot be determined without a chemical reaction.
A)
![<strong>For the rate law Rate = k[A][B]<sup>1/2</sup>,the partial order with respect to A is __________,the partial order with respect to B is __________,and the total order is __________.</strong> A) B) C) D) E)The orders cannot be determined without a chemical reaction.](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35db_6501_b44f_0b06c6070540_TB6561_11_TB6561_11.jpg)
B)
![<strong>For the rate law Rate = k[A][B]<sup>1/2</sup>,the partial order with respect to A is __________,the partial order with respect to B is __________,and the total order is __________.</strong> A) B) C) D) E)The orders cannot be determined without a chemical reaction.](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35db_8c12_b44f_d9313ff5a7ae_TB6561_11_TB6561_11.jpg)
C)
![<strong>For the rate law Rate = k[A][B]<sup>1/2</sup>,the partial order with respect to A is __________,the partial order with respect to B is __________,and the total order is __________.</strong> A) B) C) D) E)The orders cannot be determined without a chemical reaction.](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35db_8c13_b44f_e51c467dd46e_TB6561_11_TB6561_11.jpg)
D)
![<strong>For the rate law Rate = k[A][B]<sup>1/2</sup>,the partial order with respect to A is __________,the partial order with respect to B is __________,and the total order is __________.</strong> A) B) C) D) E)The orders cannot be determined without a chemical reaction.](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35db_b324_b44f_f706158a2a69_TB6561_11_TB6561_11.jpg)
E)The orders cannot be determined without a chemical reaction.

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
44
The rate of a reaction is found to triple when the concentration of one reactant is tripled.The order of the reaction with respect to this reactant is __________
A)first.
B)second.
C)zero.
D)one-half.
E)third.
A)first.
B)second.
C)zero.
D)one-half.
E)third.

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
45
Given the following data,determine the rate law for the reaction NH4+(aq)+ NO2-(aq) N2(g)+ 2 H2O( ![<strong>Given the following data,determine the rate law for the reaction NH<sub>4</sub><sup>+</sup>(aq)+ NO<sub>2</sub><sup>-</sup>(aq) \to N<sub>2</sub>(g)+ 2 H<sub>2</sub>O( ) </strong> A)k[NH<sub>4</sub><sup>+</sup>][NO<sub>2</sub><sup>-</sup>] B)k[NH<sub>4</sub><sup>+</sup>]<sup>2</sup>[NO<sub>2</sub><sup>-</sup>] C)k[NH<sub>4</sub><sup>+</sup>][NO<sub>2</sub><sup>-</sup>]<sup>1/2</sup> D)k[NH<sub>4</sub><sup>+</sup>]<sup>1/2</sup>[NO<sub>2</sub><sup>-</sup>]<sup>2</sup> E)k[NH<sub>4</sub><sup>+</sup>][NO<sub>2</sub><sup>-</sup>]<sup>2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35de_c071_b44f_7375472d3c66_TB6561_11_TB6561_11.jpg) )
)
![<strong>Given the following data,determine the rate law for the reaction NH<sub>4</sub><sup>+</sup>(aq)+ NO<sub>2</sub><sup>-</sup>(aq) \to N<sub>2</sub>(g)+ 2 H<sub>2</sub>O( ) </strong> A)k[NH<sub>4</sub><sup>+</sup>][NO<sub>2</sub><sup>-</sup>] B)k[NH<sub>4</sub><sup>+</sup>]<sup>2</sup>[NO<sub>2</sub><sup>-</sup>] C)k[NH<sub>4</sub><sup>+</sup>][NO<sub>2</sub><sup>-</sup>]<sup>1/2</sup> D)k[NH<sub>4</sub><sup>+</sup>]<sup>1/2</sup>[NO<sub>2</sub><sup>-</sup>]<sup>2</sup> E)k[NH<sub>4</sub><sup>+</sup>][NO<sub>2</sub><sup>-</sup>]<sup>2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11efd0e2_9389_4e12_bf79_5589b8c2d4e2_TB6561_00.jpg)
A)k[NH4+][NO2-]
B)k[NH4+]2[NO2-]
C)k[NH4+][NO2-]1/2
D)k[NH4+]1/2[NO2-]2
E)k[NH4+][NO2-]2
![<strong>Given the following data,determine the rate law for the reaction NH<sub>4</sub><sup>+</sup>(aq)+ NO<sub>2</sub><sup>-</sup>(aq) \to N<sub>2</sub>(g)+ 2 H<sub>2</sub>O( ) </strong> A)k[NH<sub>4</sub><sup>+</sup>][NO<sub>2</sub><sup>-</sup>] B)k[NH<sub>4</sub><sup>+</sup>]<sup>2</sup>[NO<sub>2</sub><sup>-</sup>] C)k[NH<sub>4</sub><sup>+</sup>][NO<sub>2</sub><sup>-</sup>]<sup>1/2</sup> D)k[NH<sub>4</sub><sup>+</sup>]<sup>1/2</sup>[NO<sub>2</sub><sup>-</sup>]<sup>2</sup> E)k[NH<sub>4</sub><sup>+</sup>][NO<sub>2</sub><sup>-</sup>]<sup>2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35de_c071_b44f_7375472d3c66_TB6561_11_TB6561_11.jpg) )
)![<strong>Given the following data,determine the rate law for the reaction NH<sub>4</sub><sup>+</sup>(aq)+ NO<sub>2</sub><sup>-</sup>(aq) \to N<sub>2</sub>(g)+ 2 H<sub>2</sub>O( ) </strong> A)k[NH<sub>4</sub><sup>+</sup>][NO<sub>2</sub><sup>-</sup>] B)k[NH<sub>4</sub><sup>+</sup>]<sup>2</sup>[NO<sub>2</sub><sup>-</sup>] C)k[NH<sub>4</sub><sup>+</sup>][NO<sub>2</sub><sup>-</sup>]<sup>1/2</sup> D)k[NH<sub>4</sub><sup>+</sup>]<sup>1/2</sup>[NO<sub>2</sub><sup>-</sup>]<sup>2</sup> E)k[NH<sub>4</sub><sup>+</sup>][NO<sub>2</sub><sup>-</sup>]<sup>2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11efd0e2_9389_4e12_bf79_5589b8c2d4e2_TB6561_00.jpg)
A)k[NH4+][NO2-]
B)k[NH4+]2[NO2-]
C)k[NH4+][NO2-]1/2
D)k[NH4+]1/2[NO2-]2
E)k[NH4+][NO2-]2

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
46
In a rate law,the partial orders are determined by __________
A)the reactant concentrations.
B)the stoichiometric coefficients.
C)the product concentrations.
D)experiment.
E)the difference between the forward and reverse rates.
A)the reactant concentrations.
B)the stoichiometric coefficients.
C)the product concentrations.
D)experiment.
E)the difference between the forward and reverse rates.

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following statements regarding reaction rates and kinetic molecular theory is NOT true?
A)Higher concentrations of reactants typically lead to greater reaction rates because a larger number of effective molecular collisions occur.
B)Collisions between product molecules that reform reactant molecules do not affect the overall rate of reaction.
C)The actual dependence of reaction rate on concentration must be determined experimentally because it depends on the reaction mechanism.
D)At t = 0,the maximum rate should be observed because only reactant molecules are present.
E)As the reaction proceeds,fewer reactant molecules are present and the reaction rate typically decreases.
A)Higher concentrations of reactants typically lead to greater reaction rates because a larger number of effective molecular collisions occur.
B)Collisions between product molecules that reform reactant molecules do not affect the overall rate of reaction.
C)The actual dependence of reaction rate on concentration must be determined experimentally because it depends on the reaction mechanism.
D)At t = 0,the maximum rate should be observed because only reactant molecules are present.
E)As the reaction proceeds,fewer reactant molecules are present and the reaction rate typically decreases.

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
48
The reaction,A + 2 B C,is first order in B and A.The overall order of the reaction is __________
A)first.
B)second.
C)third.
D)zero.
E)fourth.
A)first.
B)second.
C)third.
D)zero.
E)fourth.

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
49
The rate of a reaction is found to quadruple when the concentration of one reactant is doubled.The order of the reaction with respect to this reactant is __________
A)first.
B)second.
C)one-quarter.
D)one-half.
E)third.
A)first.
B)second.
C)one-quarter.
D)one-half.
E)third.

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
50
Given the following data,determine the order of the reaction with respect to ICl. H2(g)+ 2 ICl(g) I2(g)+ 2 HCl(g)
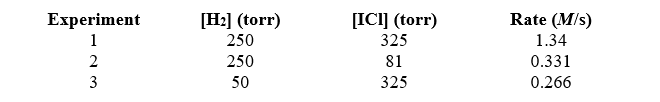
A)one-half
B)first
C)second
D)third
E)three-halves
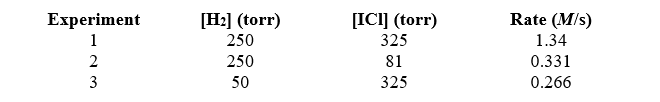
A)one-half
B)first
C)second
D)third
E)three-halves

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
51
For the rate law Rate = k[A]1/2[B],the partial order with respect to A is __________,the partial order with respect to B is __________,and the total order is __________.
A)![<strong>For the rate law Rate = k[A]<sup>1/2</sup>[B],the partial order with respect to A is __________,the partial order with respect to B is __________,and the total order is __________.</strong> A) B) C) D) E)The orders cannot be determined without a chemical reaction.](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35dc_2855_b44f_27d3d26e95bd_TB6561_11_TB6561_11.jpg)
B)![<strong>For the rate law Rate = k[A]<sup>1/2</sup>[B],the partial order with respect to A is __________,the partial order with respect to B is __________,and the total order is __________.</strong> A) B) C) D) E)The orders cannot be determined without a chemical reaction.](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35dc_4f66_b44f_638946fada4f_TB6561_11_TB6561_11.jpg)
C)![<strong>For the rate law Rate = k[A]<sup>1/2</sup>[B],the partial order with respect to A is __________,the partial order with respect to B is __________,and the total order is __________.</strong> A) B) C) D) E)The orders cannot be determined without a chemical reaction.](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35dc_4f67_b44f_990007d6f43c_TB6561_11_TB6561_11.jpg)
D)![<strong>For the rate law Rate = k[A]<sup>1/2</sup>[B],the partial order with respect to A is __________,the partial order with respect to B is __________,and the total order is __________.</strong> A) B) C) D) E)The orders cannot be determined without a chemical reaction.](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35dc_7678_b44f_d38654ad44a1_TB6561_11_TB6561_11.jpg)
E)The orders cannot be determined without a chemical reaction.
A)
![<strong>For the rate law Rate = k[A]<sup>1/2</sup>[B],the partial order with respect to A is __________,the partial order with respect to B is __________,and the total order is __________.</strong> A) B) C) D) E)The orders cannot be determined without a chemical reaction.](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35dc_2855_b44f_27d3d26e95bd_TB6561_11_TB6561_11.jpg)
B)
![<strong>For the rate law Rate = k[A]<sup>1/2</sup>[B],the partial order with respect to A is __________,the partial order with respect to B is __________,and the total order is __________.</strong> A) B) C) D) E)The orders cannot be determined without a chemical reaction.](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35dc_4f66_b44f_638946fada4f_TB6561_11_TB6561_11.jpg)
C)
![<strong>For the rate law Rate = k[A]<sup>1/2</sup>[B],the partial order with respect to A is __________,the partial order with respect to B is __________,and the total order is __________.</strong> A) B) C) D) E)The orders cannot be determined without a chemical reaction.](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35dc_4f67_b44f_990007d6f43c_TB6561_11_TB6561_11.jpg)
D)
![<strong>For the rate law Rate = k[A]<sup>1/2</sup>[B],the partial order with respect to A is __________,the partial order with respect to B is __________,and the total order is __________.</strong> A) B) C) D) E)The orders cannot be determined without a chemical reaction.](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35dc_7678_b44f_d38654ad44a1_TB6561_11_TB6561_11.jpg)
E)The orders cannot be determined without a chemical reaction.

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
52
For the rate law Rate = k[A][B]3/2,the partial order with respect to A is __________,the partial order with respect to B is __________,and the total order is __________.
A)![<strong>For the rate law Rate = k[A][B]<sup>3/2</sup>,the partial order with respect to A is __________,the partial order with respect to B is __________,and the total order is __________.</strong> A) B) C) D) E)The orders cannot be determined without a chemical reaction.](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35dc_eba9_b44f_e9e0019ea608_TB6561_11_TB6561_11.jpg)
B)![<strong>For the rate law Rate = k[A][B]<sup>3/2</sup>,the partial order with respect to A is __________,the partial order with respect to B is __________,and the total order is __________.</strong> A) B) C) D) E)The orders cannot be determined without a chemical reaction.](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35dd_12ba_b44f_69bcdddb5f41_TB6561_11_TB6561_11.jpg)
C)![<strong>For the rate law Rate = k[A][B]<sup>3/2</sup>,the partial order with respect to A is __________,the partial order with respect to B is __________,and the total order is __________.</strong> A) B) C) D) E)The orders cannot be determined without a chemical reaction.](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35dd_39cb_b44f_71d946c1e5da_TB6561_11_TB6561_11.jpg)
D)![<strong>For the rate law Rate = k[A][B]<sup>3/2</sup>,the partial order with respect to A is __________,the partial order with respect to B is __________,and the total order is __________.</strong> A) B) C) D) E)The orders cannot be determined without a chemical reaction.](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35dd_60dc_b44f_4dc9069322d8_TB6561_11_TB6561_11.jpg)
E)The orders cannot be determined without a chemical reaction.
A)
![<strong>For the rate law Rate = k[A][B]<sup>3/2</sup>,the partial order with respect to A is __________,the partial order with respect to B is __________,and the total order is __________.</strong> A) B) C) D) E)The orders cannot be determined without a chemical reaction.](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35dc_eba9_b44f_e9e0019ea608_TB6561_11_TB6561_11.jpg)
B)
![<strong>For the rate law Rate = k[A][B]<sup>3/2</sup>,the partial order with respect to A is __________,the partial order with respect to B is __________,and the total order is __________.</strong> A) B) C) D) E)The orders cannot be determined without a chemical reaction.](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35dd_12ba_b44f_69bcdddb5f41_TB6561_11_TB6561_11.jpg)
C)
![<strong>For the rate law Rate = k[A][B]<sup>3/2</sup>,the partial order with respect to A is __________,the partial order with respect to B is __________,and the total order is __________.</strong> A) B) C) D) E)The orders cannot be determined without a chemical reaction.](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35dd_39cb_b44f_71d946c1e5da_TB6561_11_TB6561_11.jpg)
D)
![<strong>For the rate law Rate = k[A][B]<sup>3/2</sup>,the partial order with respect to A is __________,the partial order with respect to B is __________,and the total order is __________.</strong> A) B) C) D) E)The orders cannot be determined without a chemical reaction.](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11eaa8e1_35dd_60dc_b44f_4dc9069322d8_TB6561_11_TB6561_11.jpg)
E)The orders cannot be determined without a chemical reaction.

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
53
Given the following data,determine the order of the reaction with respect to H2. H2(g)+ 2 ICl(g) I2(g)+ 2 HCl(g)

A)one-half
B)second
C)first
D)third
E)three-halves

A)one-half
B)second
C)first
D)third
E)three-halves

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
54
The reaction 2 NO(g)+ O2(g) 2 NO2(g)has the following rate law: Rate = k[O2][NO]2.If the concentration of NO is reduced by a factor of two,the rate will __________
A)double.
B)quadruple.
C)decrease by a factor of one-fourth.
D)decrease by a factor of one-half.
E)remain the same.
A)double.
B)quadruple.
C)decrease by a factor of one-fourth.
D)decrease by a factor of one-half.
E)remain the same.

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
55
Given the following data,determine the order of the reaction with respect to Cl2. CO(g)+ Cl2(g) COCl2(g)

A)one-half
B)first
C)second
D)third
E)three-halves

A)one-half
B)first
C)second
D)third
E)three-halves

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
56
The reaction CHCl3(g)+ Cl2(g) CCl4(g)+ HCl(g)has the following rate law: Rate = k[CHCl3][Cl2].If the concentration of CHCl3 is increased by a factor of five while the concentration of Cl2 is kept the same,the rate will __________
A)double.
B)triple.
C)stay the same.
D)increase by a factor of five.
E)decrease by a factor of one-fifth.
A)double.
B)triple.
C)stay the same.
D)increase by a factor of five.
E)decrease by a factor of one-fifth.

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
57
Given the following data,determine the order of the reaction with respect to Cl2. 2 NO(g)+ Cl2(g) 2 NOCl(g)
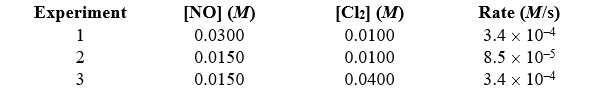
A)first
B)second
C)third
D)fourth
E)zero
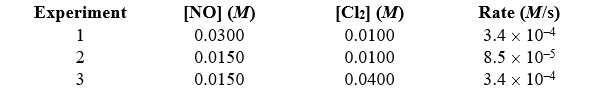
A)first
B)second
C)third
D)fourth
E)zero

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
58
Determine the overall order of the reaction 2 NO(g)+ Cl2(g) 2 NOCl(g)from the following data:
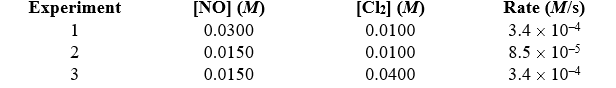
A)first
B)second
C)third
D)three-halves
E)fourth
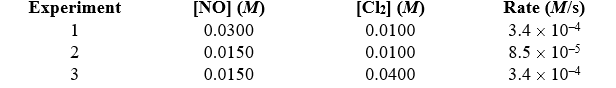
A)first
B)second
C)third
D)three-halves
E)fourth

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
59
The rate of disappearance of HI in the reaction 2 HI(g) I2(g)+ H2(g)is shown in the following figure.Estimate the initial rate of reaction. 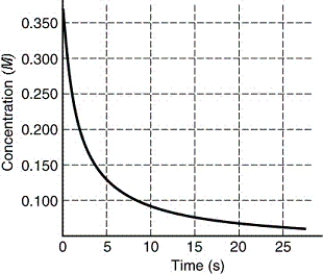
A)0.350 M/s
B)0.140 M/s
C)0.014 M/s
D)0.175 M/s
E)0.070 M/s

A)0.350 M/s
B)0.140 M/s
C)0.014 M/s
D)0.175 M/s
E)0.070 M/s

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
60
Given the following data,determine the order of the reaction with respect to NO(g). 2 NO(g)+ Cl2(g) 2 NOCl(g)

A)first
B)second
C)third
D)fourth
E)zero

A)first
B)second
C)third
D)fourth
E)zero

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
61
Which of these could be the units of the rate constant for a first-order reaction?
A)M/s
B)1/Ms
C)1/Ms2
D)1/s
E)Ms
A)M/s
B)1/Ms
C)1/Ms2
D)1/s
E)Ms

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
62
Which of these could be the units for the rate constant in a second-order reaction?
A)M -1 s
B)M s-1
C)M -1 s-1
D)M -2 s-1
E)Ms
A)M -1 s
B)M s-1
C)M -1 s-1
D)M -2 s-1
E)Ms

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
63
Suppose 2.00 g azomethane (58.1 g/mol)are placed in a 2.50 L container at 425°C and allowed to decompose according to the first order reaction,CH3NNCH3(g) C2H6(g)+ N2(g).Initially,0.0234 moles of C2H6 are produced per second.What is the rate constant?
A)0.588 s-1
B)0.0468 ss-1
C)0.0138 s-1
D)0.0344 s-1
E)0.680 s-1
A)0.588 s-1
B)0.0468 ss-1
C)0.0138 s-1
D)0.0344 s-1
E)0.680 s-1

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
64
Given the following data,determine the rate law for the reaction CH3CHO(g) CH4(g)+ CO(g)
![<strong>Given the following data,determine the rate law for the reaction CH<sub>3</sub>CHO(g) \to CH<sub>4</sub>(g)+ CO(g) </strong> A)k B)kP[CH<sub>3</sub>CHO] C)kP[CH<sub>3</sub>CHO]<sup>3/2</sup> D)kP[CH<sub>3</sub>CHO]<sup>1/2</sup> E)kP[CH<sub>3</sub>CHO]<sup>2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11efd0e3_5e98_0839_bf79_ebbedc751be4_TB6561_00.jpg)
A)k
B)kP[CH3CHO]
C)kP[CH3CHO]3/2
D)kP[CH3CHO]1/2
E)kP[CH3CHO]2
![<strong>Given the following data,determine the rate law for the reaction CH<sub>3</sub>CHO(g) \to CH<sub>4</sub>(g)+ CO(g) </strong> A)k B)kP[CH<sub>3</sub>CHO] C)kP[CH<sub>3</sub>CHO]<sup>3/2</sup> D)kP[CH<sub>3</sub>CHO]<sup>1/2</sup> E)kP[CH<sub>3</sub>CHO]<sup>2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11efd0e3_5e98_0839_bf79_ebbedc751be4_TB6561_00.jpg)
A)k
B)kP[CH3CHO]
C)kP[CH3CHO]3/2
D)kP[CH3CHO]1/2
E)kP[CH3CHO]2

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
65
The reaction C2H5Cl(g) C2H4(g)+ HCl(g)has a rate constant of approximately 4.20 10-5 s-1 at 700 K.What is the reaction rate if 123.8 g C2H5Cl is placed in an 8.00 L container at 700 K?
A)1.75 10-4 M/s
B)3.36 10-4 M/s
C)6.50 10-4 M/s
D)1.01 10-5 M/s
E)8.01 10-5 M/s
A)1.75 10-4 M/s
B)3.36 10-4 M/s
C)6.50 10-4 M/s
D)1.01 10-5 M/s
E)8.01 10-5 M/s

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following plots indicates that the reaction is zero order?
A)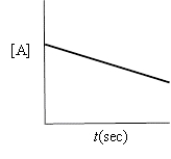
B)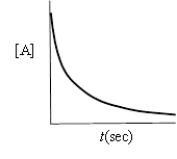
C)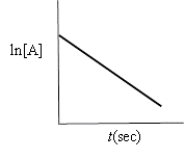
D)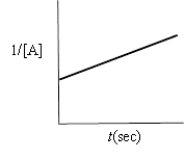
E)none of these
A)

B)

C)

D)

E)none of these

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
67
The initial rate data for the reaction 2 N2O5(g) 4 NO2(g)+ O2(g)is shown in the following table.Determine the value of the rate constant for this reaction.

A)4.09 s-1
B)0.176 s-1
C)0.0569 s-1
D)0.225 s-1
E)80.1 s-1

A)4.09 s-1
B)0.176 s-1
C)0.0569 s-1
D)0.225 s-1
E)80.1 s-1

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
68
Determine the overall order of the reaction: H2(g)+ 2 ICl(g) I2(g)+ 2 HCl(g)from the following data:

A)first
B)second
C)third
D)fourth
E)zero

A)first
B)second
C)third
D)fourth
E)zero

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
69
Given the following data,determine the rate law for the reaction H2(g)+ 2 ICl(g) I2(g)+ 2 HCl(g)
![<strong>Given the following data,determine the rate law for the reaction H<sub>2</sub>(g)+ 2 ICl(g) \to I<sub>2</sub>(g)+ 2 HCl(g) </strong> A)k[H<sub>2</sub>][ICl] B)k[H<sub>2</sub>][ICl]<sup>2</sup> C)k[H<sub>2</sub>]<sup>2</sup>[ICl] D)k[H<sub>2</sub>]<sup>1/2</sup>[ICl]<sup>1/2</sup> E)k[H<sub>2</sub>][ICl]<sup>1/2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11efd0e3_42df_5268_bf79_096203742045_TB6561_11.jpg)
A)k[H2][ICl]
B)k[H2][ICl]2
C)k[H2]2[ICl]
D)k[H2]1/2[ICl]1/2
E)k[H2][ICl]1/2
![<strong>Given the following data,determine the rate law for the reaction H<sub>2</sub>(g)+ 2 ICl(g) \to I<sub>2</sub>(g)+ 2 HCl(g) </strong> A)k[H<sub>2</sub>][ICl] B)k[H<sub>2</sub>][ICl]<sup>2</sup> C)k[H<sub>2</sub>]<sup>2</sup>[ICl] D)k[H<sub>2</sub>]<sup>1/2</sup>[ICl]<sup>1/2</sup> E)k[H<sub>2</sub>][ICl]<sup>1/2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11efd0e3_42df_5268_bf79_096203742045_TB6561_11.jpg)
A)k[H2][ICl]
B)k[H2][ICl]2
C)k[H2]2[ICl]
D)k[H2]1/2[ICl]1/2
E)k[H2][ICl]1/2

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
70
Given the following data,determine the rate constant,k,of the reaction: H2(g)+ 2 ICl(g) I2(g)+ 2 HCl(g)
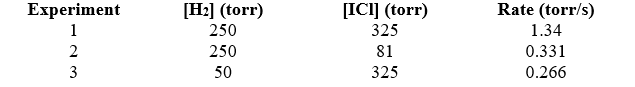
A)1.65 10-5 torr-1s-1
B)6.06 104 torr-1s-1
C)8.17 10-5 torr-1s-1
D)1.34 torr-1s-1
E)3.48 103 torr-1s-1
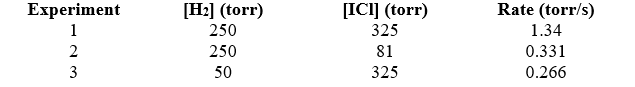
A)1.65 10-5 torr-1s-1
B)6.06 104 torr-1s-1
C)8.17 10-5 torr-1s-1
D)1.34 torr-1s-1
E)3.48 103 torr-1s-1

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
71
Determine the overall order of reaction for CO(g)+ Cl2(g) COCl2(g)from the following data:

A)one-half
B)first
C)second
D)third
E)three-halves

A)one-half
B)first
C)second
D)third
E)three-halves

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
72
If the reaction 3 A + B +  C 2 D + E is first order overall,which of these could be the units of its rate constant,k?
C 2 D + E is first order overall,which of these could be the units of its rate constant,k?
A)1/s
B)M/s
C)1/Ms
D)1/Ms2
E)Ms
 C 2 D + E is first order overall,which of these could be the units of its rate constant,k?
C 2 D + E is first order overall,which of these could be the units of its rate constant,k?A)1/s
B)M/s
C)1/Ms
D)1/Ms2
E)Ms

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
73
Given the following data,determine the rate constant of the reaction 2 NO(g)+ Cl2(g) 2 NOCl(g)
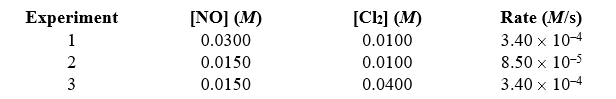
A)1.13 M -2s-1
B)9.44 M -2s-1
C)37.8 M -2s-1
D)0.0265 M -2s-1
E)59.6 M -2s-1
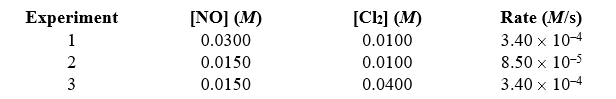
A)1.13 M -2s-1
B)9.44 M -2s-1
C)37.8 M -2s-1
D)0.0265 M -2s-1
E)59.6 M -2s-1

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following statements regarding rate laws and rate constants is true?
A)The rate constant is independent of temperature.
B)The units of the rate constant depend on the stoichiometry of the reaction.
C)The rate constant typically depends on concentration of reactant(s).
D)The rate typically depends on concentration of reactant(s).
E)The rate constant does not change when a catalyst is introduced.
A)The rate constant is independent of temperature.
B)The units of the rate constant depend on the stoichiometry of the reaction.
C)The rate constant typically depends on concentration of reactant(s).
D)The rate typically depends on concentration of reactant(s).
E)The rate constant does not change when a catalyst is introduced.

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following plots indicates the reaction is second order?
A)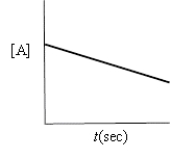
B)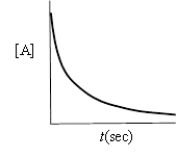
C)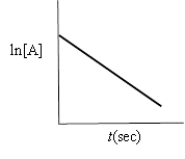
D)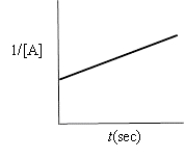
E)none of these
A)

B)

C)

D)

E)none of these

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
76
To determine the rate law for a reaction,data from several reactions run at different concentrations are usually required.For what type of reaction are data from just one experiment plotting concentration versus time sufficient to determine the rate law?
A)zero-order reactions
B)first-order reactions
C)second-order reactions
D)reactions with fractional orders
E)photochemical reactions
A)zero-order reactions
B)first-order reactions
C)second-order reactions
D)reactions with fractional orders
E)photochemical reactions

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
77
Indicate which of the following plots would be obtained for a second-order reaction.
A)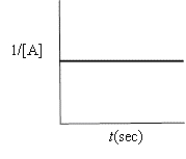
B)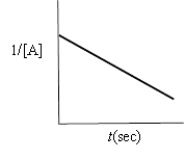
C)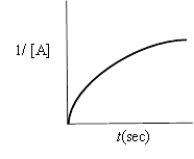
D)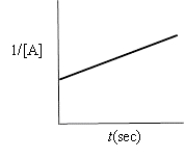
E)none of these
A)

B)

C)

D)

E)none of these

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
78
Determine the overall order of the reaction CH3CHO (g) CH4(g)+ CO(g)from the following data:

A)one-half
B)first
C)second
D)three-halves
E)third

A)one-half
B)first
C)second
D)three-halves
E)third

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
79
Given the following data,determine the rate law for the reaction 2 NO(g)+ Cl2(g) 2 NOCl(g)
![<strong>Given the following data,determine the rate law for the reaction 2 NO(g)+ Cl<sub>2</sub>(g) \to 2 NOCl(g) </strong> A)k[NO][Cl<sub>2</sub>] B)k[NO][Cl<sub>2</sub>]<sup>2</sup> C)k[NO]<sup>2</sup>[Cl<sub>2</sub>] D)k[NO]<sup>2</sup>[Cl<sub>2</sub>]<sup>2</sup> E)k[NO][Cl<sub>2</sub>]<sup>1/2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11efd0e3_26a1_b527_bf79_73b6f5f6f2d1_TB6561_00.jpg)
A)k[NO][Cl2]
B)k[NO][Cl2]2
C)k[NO]2[Cl2]
D)k[NO]2[Cl2]2
E)k[NO][Cl2]1/2
![<strong>Given the following data,determine the rate law for the reaction 2 NO(g)+ Cl<sub>2</sub>(g) \to 2 NOCl(g) </strong> A)k[NO][Cl<sub>2</sub>] B)k[NO][Cl<sub>2</sub>]<sup>2</sup> C)k[NO]<sup>2</sup>[Cl<sub>2</sub>] D)k[NO]<sup>2</sup>[Cl<sub>2</sub>]<sup>2</sup> E)k[NO][Cl<sub>2</sub>]<sup>1/2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB6561/11efd0e3_26a1_b527_bf79_73b6f5f6f2d1_TB6561_00.jpg)
A)k[NO][Cl2]
B)k[NO][Cl2]2
C)k[NO]2[Cl2]
D)k[NO]2[Cl2]2
E)k[NO][Cl2]1/2

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following plots would indicate that a reaction was first order?
A)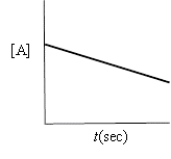
B)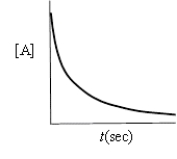
C)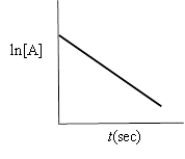
D)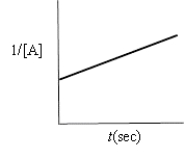
E)none of these
A)

B)

C)

D)

E)none of these

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck



