Deck 10: Liquids and Solids
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/126
Play
Full screen (f)
Deck 10: Liquids and Solids
1
Which of the following should have the lowest boiling point?
A)Na2S
B)HF
C)NH3
D)N2
E)H2O
A)Na2S
B)HF
C)NH3
D)N2
E)H2O
N2
2
Which of the species below would you expect to show the least hydrogen bonding?
A)NH3
B)H2O
C)HF
D)CH4
E)all the same
A)NH3
B)H2O
C)HF
D)CH4
E)all the same
CH4
3
The elements of group 5A,the nitrogen family,form compounds with hydrogen having the boiling points listed below: SbH3 -17°C,AsH3 -55°C,PH3 -87°C,NH3 -33°C
The first three compounds illustrate a trend where the boiling point decreases as the mass decreases;however,ammonia (NH3)does not follow the trend because of
A)dipole-dipole attraction
B)metallic bonding
C)hydrogen bonding
D)London dispersion forces
E)ionic bonding
The first three compounds illustrate a trend where the boiling point decreases as the mass decreases;however,ammonia (NH3)does not follow the trend because of
A)dipole-dipole attraction
B)metallic bonding
C)hydrogen bonding
D)London dispersion forces
E)ionic bonding
hydrogen bonding
4
Which drawing best represents the interactions in a sample of HF?
A)I
B)II
C)III
D)I,II
E)all of the above
A)I
B)II
C)III
D)I,II
E)all of the above

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
5
Intermolecular forces are weaker than intramolecular bonds.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
6
The freezing point of helium is -270°C.The freezing point of xenon is -112°C.Both of these are in the noble gas family.Which of the following statements is supported by these data?
A)Helium and xenon form highly polar molecules.
B)As the molecular weight of the noble gas increases,the freezing point decreases.
C)The London dispersion forces between the helium molecules are greater than the London dispersion between the xenon molecules.
D)The London dispersion forces between the helium molecules are less than the London dispersion forces between the xenon molecules.
E)None of these.
A)Helium and xenon form highly polar molecules.
B)As the molecular weight of the noble gas increases,the freezing point decreases.
C)The London dispersion forces between the helium molecules are greater than the London dispersion between the xenon molecules.
D)The London dispersion forces between the helium molecules are less than the London dispersion forces between the xenon molecules.
E)None of these.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
7
In which of the following groups of substances would dispersion forces be the only significant factors in determining boiling points? I.Cl2
II.HF
III.Ne
IV.KNO2
V.CCl4
A)I,III,V
B)I,II,III
C)II,IV
D)II,V
E)III,IV,V
II.HF
III.Ne
IV.KNO2
V.CCl4
A)I,III,V
B)I,II,III
C)II,IV
D)II,V
E)III,IV,V

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following statements are incorrect concerning drawing III?
A)Electrostatic interactions exist between the molecules.
B)The molecules find the best compromise between attraction and repulsion.
C)These molecules exhibit ionic bonding.
D)OCS exhibits this type of interaction.
E)Two of the above statements are incorrect.
A)Electrostatic interactions exist between the molecules.
B)The molecules find the best compromise between attraction and repulsion.
C)These molecules exhibit ionic bonding.
D)OCS exhibits this type of interaction.
E)Two of the above statements are incorrect.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
9
The bonds between hydrogen and oxygen within a water molecule can be characterized as __________.
A)hydrogen bonds
B)London dispersion forces
C)intermolecular forces
D)intramolecular forces
E)dispersion forces
A)hydrogen bonds
B)London dispersion forces
C)intermolecular forces
D)intramolecular forces
E)dispersion forces

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
10
When a water molecule forms a hydrogen bond with another water molecule,which atoms are involved in the interaction?
A)a hydrogen from one molecule and a hydrogen from the other molecule
B)a hydrogen from one molecule and an oxygen from the other molecule
C)an oxygen from one molecule and an oxygen from the other molecule
D)an oxygen and a hydrogen from the same molecule
E)two hydrogens from one molecule and one hydrogen from the other molecule
A)a hydrogen from one molecule and a hydrogen from the other molecule
B)a hydrogen from one molecule and an oxygen from the other molecule
C)an oxygen from one molecule and an oxygen from the other molecule
D)an oxygen and a hydrogen from the same molecule
E)two hydrogens from one molecule and one hydrogen from the other molecule

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is the correct order of boiling points for KNO3,CH3OH,C2H6,Ne?
A)Ne < CH3OH < C2H6 < KNO3
B)KNO3 < CH3OH < C2H6 < Ne
C)Ne < C2H6 < KNO3 < CH3OH
D)Ne < C2H6 < CH3OH < KNO3
E)C2H6 < Ne < CH3OH < KNO3
A)Ne < CH3OH < C2H6 < KNO3
B)KNO3 < CH3OH < C2H6 < Ne
C)Ne < C2H6 < KNO3 < CH3OH
D)Ne < C2H6 < CH3OH < KNO3
E)C2H6 < Ne < CH3OH < KNO3

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
12
Hydrogen bonding is a type of London dispersion force.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
13
In general,the density of a compound as a gas is closer in value to that of the compound as a liquid than the density of the compound as a liquid is in value to that of the compound as a solid.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
14
Consider the representations below to answer the next three questions.
(I)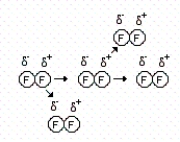 (II)
(II)  (III)
(III) 
How many of the following statements are correct concerning drawing I?
I.Each molecule induces a dipole onto the next molecule in close proximity.
II.The phenomenon shown is relatively weak and short-lived.
III.C8H18 contains this type of interaction.
IV.The forces that exist in this example are London dispersion forces.
A)0
B)1
C)2
D)3
E)4
(I)
 (II)
(II)  (III)
(III) 
How many of the following statements are correct concerning drawing I?
I.Each molecule induces a dipole onto the next molecule in close proximity.
II.The phenomenon shown is relatively weak and short-lived.
III.C8H18 contains this type of interaction.
IV.The forces that exist in this example are London dispersion forces.
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following would you expect to have the highest boiling point?
A)F2
B)Cl2
C)Br2
D)I2
E)All of the above have the same boiling point.
A)F2
B)Cl2
C)Br2
D)I2
E)All of the above have the same boiling point.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
16
Order the intermolecular forces (dipole-dipole,London dispersion,ionic,and hydrogen-bonding)from weakest to strongest .
A)dipole-dipole,London dispersion,ionic,and hydrogen-bonding
B)London dispersion,dipole-dipole,hydrogen-bonding,and ionic
C)hydrogen-bonding,dipole-dipole,London dispersion,and ionic
D)dipole-dipole,ionic,London dispersion,and hydrogen-bonding
E)London dispersion,ionic,dipole-dipole,and hydrogen-bonding
A)dipole-dipole,London dispersion,ionic,and hydrogen-bonding
B)London dispersion,dipole-dipole,hydrogen-bonding,and ionic
C)hydrogen-bonding,dipole-dipole,London dispersion,and ionic
D)dipole-dipole,ionic,London dispersion,and hydrogen-bonding
E)London dispersion,ionic,dipole-dipole,and hydrogen-bonding

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
17
Second row hydrides generally have higher than expected boiling points for their position on the periodic table.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
18
Hydrogen bonds account for which of the following observation?
A)Hydrogen naturally exists as a diatomic molecule.
B)Hydrogen is easily combustible with oxygen.
C)Water molecules are bent or "V-shaped."
D)Air is more dense than hydrogen gas.
E)For its molar mass,water has a high boiling point.
A)Hydrogen naturally exists as a diatomic molecule.
B)Hydrogen is easily combustible with oxygen.
C)Water molecules are bent or "V-shaped."
D)Air is more dense than hydrogen gas.
E)For its molar mass,water has a high boiling point.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
19
Which substance involves no bonding forces except London dispersion forces?
A)NaCl(l)
B)HF(l)
C)N2(s)
D)H2O(l)
E)K(s)
A)NaCl(l)
B)HF(l)
C)N2(s)
D)H2O(l)
E)K(s)

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
20
The molecules in a sample of solid SO2 are attracted to each other by a combination of
A)London forces and H-bonding
B)H-bonding and ionic bonding
C)covalent bonding and dipole-dipole interactions
D)London forces and dipole-dipole interactions
E)none of these
A)London forces and H-bonding
B)H-bonding and ionic bonding
C)covalent bonding and dipole-dipole interactions
D)London forces and dipole-dipole interactions
E)none of these

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
21
The molar volume of a certain form of solid lead is 18 cm3/mol.Assuming cubic closest packed structure,determine the following:
The number of Pb atoms per unit cell.
A)1
B)2
C)4
D)6
E)10
The number of Pb atoms per unit cell.
A)1
B)2
C)4
D)6
E)10

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
22
Cubic closest packing is another name for _____________.
A)simple cubic packing
B)body-centered cubic packing
C)face-centered cubic packing
D)hexagonal closest packing
E)more than one of these
A)simple cubic packing
B)body-centered cubic packing
C)face-centered cubic packing
D)hexagonal closest packing
E)more than one of these

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
23
The radius of a Pb atom.
A)1.74 pm
B)17.4 pm
C)174 pm
D)1740 pm
E)none of these
A)1.74 pm
B)17.4 pm
C)174 pm
D)1740 pm
E)none of these

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
24
A crystal was analyzed with x-rays having 2.33 Å wavelength.The angle of first-order diffraction (n = 1)was 19.2°.What would be the angle for second-order diffraction (n = 2)?
A)38.4 Å
B)41.1 Å
C)9.46 Å
D)14.2 Å
E)4.72 Å
A)38.4 Å
B)41.1 Å
C)9.46 Å
D)14.2 Å
E)4.72 Å

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following compounds has the lowest viscosity?
A)CCl4(l)
B)N2(g)
C)H2O(l)
D)CH3-(CH2)25-CH3(l)
E)HCl(g)
A)CCl4(l)
B)N2(g)
C)H2O(l)
D)CH3-(CH2)25-CH3(l)
E)HCl(g)

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
26
In any cubic lattice an atom lying at the corner of a unit cell is shared equally by how many unit cells?
A)1
B)4
C)8
D)2
E)6
A)1
B)4
C)8
D)2
E)6

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following statements about liquids is true?
A)Droplet formation occurs because of the higher stability associated with increased surface area.
B)Substances that can form hydrogen bonds will display lower melting points than predicted from periodic trends.
C)London dispersion forces arise from a distortion of the electron clouds within a molecule or atom.
D)Liquid rise within a capillary tube because of the small size lowers the effective atmospheric pressure over the surface of the liquid.
E)The boiling point of a solution is dependent solely on the atmospheric pressure over the solution.
A)Droplet formation occurs because of the higher stability associated with increased surface area.
B)Substances that can form hydrogen bonds will display lower melting points than predicted from periodic trends.
C)London dispersion forces arise from a distortion of the electron clouds within a molecule or atom.
D)Liquid rise within a capillary tube because of the small size lowers the effective atmospheric pressure over the surface of the liquid.
E)The boiling point of a solution is dependent solely on the atmospheric pressure over the solution.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
28
Liquids with large intermolecular forces tend to have high surface tension.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following is paired incorrectly?
A)crystalline solids-highly regular arrangement of their components
B)amorphous solids-considerable disorder in their structures
C)unit cell-the smallest repeating unit of the lattice
D)gold metal-simple cubic unit cell
E)glass-amorphous solid
A)crystalline solids-highly regular arrangement of their components
B)amorphous solids-considerable disorder in their structures
C)unit cell-the smallest repeating unit of the lattice
D)gold metal-simple cubic unit cell
E)glass-amorphous solid

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
30
Which one of the following statements about solid Cu (face-centered cubic unit cell)is incorrect?
A)It will conduct electricity.
B)There are two atoms per unit cell.
C)The number of atoms surrounding each Cu atom is 12.
D)The solid has a cubic closest-packed structure.
E)The length of a face diagonal is four times the Cu radius.
A)It will conduct electricity.
B)There are two atoms per unit cell.
C)The number of atoms surrounding each Cu atom is 12.
D)The solid has a cubic closest-packed structure.
E)The length of a face diagonal is four times the Cu radius.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following statements is (are)false?
I.The layering in a hexagonal closest-packed structure is aba.
II.A body-centered cubic unit cell has four atoms per unit cell.
III.For unit cells having the same edge length,a simple cubic structure would have a smaller density than a body-centered cube.
IV.Atoms in a solid consisting of only one element would have six nearest neighbors if the crystal structure were a simple cubic array.
A)I
B)II
C)II,III
D)I,IV
E)II,III,IV
I.The layering in a hexagonal closest-packed structure is aba.
II.A body-centered cubic unit cell has four atoms per unit cell.
III.For unit cells having the same edge length,a simple cubic structure would have a smaller density than a body-centered cube.
IV.Atoms in a solid consisting of only one element would have six nearest neighbors if the crystal structure were a simple cubic array.
A)I
B)II
C)II,III
D)I,IV
E)II,III,IV

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
32
Methane (CH4)exhibits stronger hydrogen bond interactions than ammonia (NH3).

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
33
When a nonpolar liquid displays a convex meniscus,which of the following explains this behavior?
A)It has a low surface tension,and therefore clings to the glass.
B)The cohesive forces are stronger than the adhesive forces to the glass.
C)The adhesive forces to the glass are stronger than the cohesive forces.
D)The liquid's viscosity is low.
E)None of these.
A)It has a low surface tension,and therefore clings to the glass.
B)The cohesive forces are stronger than the adhesive forces to the glass.
C)The adhesive forces to the glass are stronger than the cohesive forces.
D)The liquid's viscosity is low.
E)None of these.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
34
Atomic solids generally have low melting points.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
35
Chromium metal crystallizes as a body-centered cubic lattice.If the atomic radius of Cr is 1.25 angstroms,what is the density of Cr metal in g/cm3?
A)5.52
B)7.18
C)14.4
D)2.76
E)3.59
A)5.52
B)7.18
C)14.4
D)2.76
E)3.59

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
36
Aluminum metal crystallizes in a face-centered cubic structure.The relationship between the radius of an Al atom (r)and the length of an edge of the unit cell (E)is:
A)r = E/2
B)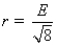
C)
D)r = 2E
E)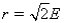
A)r = E/2
B)
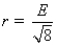
C)

D)r = 2E
E)
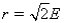

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
37
Table salt and table sugar are both crystalline solids.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
38
A crystal was analyzed with x-rays having 1.57 Å wavelength.A reflection was produced at = 21.9°.Assuming n = 1,what is the distance between the layers of atoms in the crystal?
A)8.42 Å
B)4.21 Å
C)0.475 Å
D)0.846 Å
E)2.10 Å
A)8.42 Å
B)4.21 Å
C)0.475 Å
D)0.846 Å
E)2.10 Å

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
39
The volume of a single cell.
A)1.20 102 pm3
B)1.20 104 pm3
C)1.20 106 pm3
D)1.20 108 pm3
E)none of these
A)1.20 102 pm3
B)1.20 104 pm3
C)1.20 106 pm3
D)1.20 108 pm3
E)none of these

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
40
What is responsible for capillary action,a property of liquids?
A)surface tension
B)cohesive forces
C)adhesive forces
D)viscosity
E)two of these
A)surface tension
B)cohesive forces
C)adhesive forces
D)viscosity
E)two of these

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
41
What is the simplest formula of a solid containing A,B,and C atoms in a cubic lattice in which the A atoms occupy the corners,the B atoms the body-center position,and the C atoms the faces of the unit cell?
A)ABC
B)ABC3
C)ABC6
D)A8BC6
E)A4BC3
A)ABC
B)ABC3
C)ABC6
D)A8BC6
E)A4BC3

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
42
Ice is a molecular solid.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
43
Steel is a substitutional alloy.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the compounds below is not an example of a molecular solid?
A)I2(s)
B)SiO2(s)
C)CO2(s)
D)H2O(s)
E)C25H52(s)
A)I2(s)
B)SiO2(s)
C)CO2(s)
D)H2O(s)
E)C25H52(s)

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
45
You are given a small bar of an unknown metal,M.You find the density of the metal to be 10.5 g/cm3.An X-ray diffraction experiment measures the edge of the unit cell as 409 pm.Assuming that the metal crystallizes in a face-centered cubic lattice,what is M most likely to be?
A)Ag
B)Rh
C)Pt
D)Pb
E)none of these
A)Ag
B)Rh
C)Pt
D)Pb
E)none of these

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
46
If equal,rigid spheres are arranged in a simple cubic lattice in the usual way r3,with = 3.14.]
A)0.52
B)0.32
C)0.68
D)0.48
E)none of these
A)0.52
B)0.32
C)0.68
D)0.48
E)none of these

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
47
At room temperature,CsF is expected to be
A)a gas
B)a conducting solid
C)a liquid
D)a brittle solid
E)a soft solid
A)a gas
B)a conducting solid
C)a liquid
D)a brittle solid
E)a soft solid

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following statements is true about p-type silicon?
A)It is produced by doping Si with P or As.
B)Protons are the mobile charge carriers.
C)It does not conduct electricity as well as pure Si.
D)All are true.
E)None is true.
A)It is produced by doping Si with P or As.
B)Protons are the mobile charge carriers.
C)It does not conduct electricity as well as pure Si.
D)All are true.
E)None is true.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the compounds below is an example of a network solid?
A)S8(s)
B)SiO2(s)
C)MgO(s)
D)NaCl(s)
E)C25H52(s)
A)S8(s)
B)SiO2(s)
C)MgO(s)
D)NaCl(s)
E)C25H52(s)

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
50
Steel is considered to be a(n)__________.
A)interstitial alloy
B)ionic solid
C)molecular solid
D)substitutional alloy
E)two of these
A)interstitial alloy
B)ionic solid
C)molecular solid
D)substitutional alloy
E)two of these

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
51
Silver chloride crystallizes with the sodium chloride (rock salt)structure.The length of a unit cell edge is 555 pm.What is the density of AgCl?
A)5.57 g/cm3
B)4.19 g/cm3
C)2.79 g/cm3
D)2.10 g/cm3
E)1.39 g/cm3
A)5.57 g/cm3
B)4.19 g/cm3
C)2.79 g/cm3
D)2.10 g/cm3
E)1.39 g/cm3

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
52
A material is made from Al,Ga,and As.The mole fraction of each element is 0.25,0.26,and 0.49,respectively.This material would be
A)a metallic conductor because Al is present
B)an insulator
C)a p-type semiconductor
D)an n-type semiconductor
E)none of the above
A)a metallic conductor because Al is present
B)an insulator
C)a p-type semiconductor
D)an n-type semiconductor
E)none of the above

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
53
Which of these statements is incorrect?
A)Molecular solids have high melting points.
B)The binding forces in a molecular solid include London dispersion forces.
C)Ionic solids have high melting points.
D)Ionic solids are insulators.
E)All of the statements (A-D)are correct.
A)Molecular solids have high melting points.
B)The binding forces in a molecular solid include London dispersion forces.
C)Ionic solids have high melting points.
D)Ionic solids are insulators.
E)All of the statements (A-D)are correct.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
54
Which substance can be described as cations bonded together by mobile electrons?
A)Ag(s)
B)S8(s)
C)Kr(l)
D)KCl(s)
E)HCl(l)
A)Ag(s)
B)S8(s)
C)Kr(l)
D)KCl(s)
E)HCl(l)

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
55
A solid material consists of an array of A atoms with Z atoms located in the spaces in the lattice.This solid would be classified as
A)an amalgam
B)an interstitial alloy
C)a substitutional alloy
D)a semiconductor
E)ceramic
A)an amalgam
B)an interstitial alloy
C)a substitutional alloy
D)a semiconductor
E)ceramic

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
56
Doping Se with B would produce a(n)__________ semiconductor with __________ conductivity compared to pure Se.
A)p-type,increased
B)n-type,decreased
C)n-type,increased
D)p-type,decreased
E)intrinsic,identical
A)p-type,increased
B)n-type,decreased
C)n-type,increased
D)p-type,decreased
E)intrinsic,identical

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
57
The net number of spheres in the face-centered cubic unit cell is 4.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
58
A metal crystallizes with a face-centered cubic lattice.The edge of the unit cell is 395 pm.The diameter of the metal atom is:
A)140 pm
B)198 pm
C)279 pm
D)395 pm
E)none of these
A)140 pm
B)198 pm
C)279 pm
D)395 pm
E)none of these

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following statements about steel is false?
A)It contains carbon atoms in the holes of its iron crystals.
B)The presence of carbon-iron bonds in the alloy make steel harder and stronger than pure iron.
C)Pure iron is relatively soft and ductile because it lacks directional bonding.
D)The amount of carbon directly affects the properties of steel.
E)All of these are true.
A)It contains carbon atoms in the holes of its iron crystals.
B)The presence of carbon-iron bonds in the alloy make steel harder and stronger than pure iron.
C)Pure iron is relatively soft and ductile because it lacks directional bonding.
D)The amount of carbon directly affects the properties of steel.
E)All of these are true.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
60
A metal crystallizes in a body-centered unit cell with an edge length of 2.00 102 pm.Assume the atoms in the cell touch along the cube diagonal.The percentage of empty volume in the unit cell will be:
A)0%
B)26.0%
C)32.0%
D)68.0%
E)none of these
A)0%
B)26.0%
C)32.0%
D)68.0%
E)none of these

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
61
A certain metal fluoride crystallizes in such a way that the fluoride ions occupy simple cubic lattice sites,while the metal atoms occupy the body centers of half the cubes.The formula for the metal fluoride is:
A)MF2
B)M2F
C)MF
D)MF8
E)none of these
A)MF2
B)M2F
C)MF
D)MF8
E)none of these

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
62
At normal atmospheric pressure and a temperature of 0°C,which phase(s)of H2O can exist?
A)ice and water
B)ice and water vapor
C)water only
D)water vapor only
E)ice only
A)ice and water
B)ice and water vapor
C)water only
D)water vapor only
E)ice only

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
63
The process of condensation happens when which of the following occurs?
A)A solid becomes a liquid.
B)A liquid becomes a solid.
C)A gas becomes a liquid.
D)A liquid becomes a gas.
E)A gas becomes a solid.
A)A solid becomes a liquid.
B)A liquid becomes a solid.
C)A gas becomes a liquid.
D)A liquid becomes a gas.
E)A gas becomes a solid.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
64
On a relative basis,the weaker the intermolecular forces in a substance,
A)the greater its heat of vaporization
B)the more it deviates from ideal gas behavior
C)the greater its vapor pressure at a particular temperature
D)the higher its melting point
E)none of these
A)the greater its heat of vaporization
B)the more it deviates from ideal gas behavior
C)the greater its vapor pressure at a particular temperature
D)the higher its melting point
E)none of these

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
65
Solid MgO has the same crystal structure as NaCl.How many oxide ions surround each Mg2+ ion as nearest neighbors in MgO?
A)4
B)6
C)8
D)12
E)none of these
A)4
B)6
C)8
D)12
E)none of these

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
66
An atom in an octahedral hole is surrounded by ____ atoms.
A)2
B)3
C)4
D)6
E)8
A)2
B)3
C)4
D)6
E)8

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
67
Sodium oxide (Na2O)crystallizes in a structure in which the O2- ions are in a face-centered cubic lattice and the Na+ ions are in tetrahedral holes.The number of Na+ ions in the unit cell is:
A)2
B)4
C)6
D)8
E)none of these
A)2
B)4
C)6
D)8
E)none of these

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
68
In the unit cell of sphalerite,Zn2+ ions occupy half the tetrahedral holes in a face-centered cubic lattice of S2- ions.The number of formula units of ZnS in the unit cell is:
A)6
B)4
C)3
D)2
E)1
A)6
B)4
C)3
D)2
E)1

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
69
Which of these statements is false?
A)Diamond is a covalent crystal.
B)The size of the unit cell of Li and Cs is the same.
C)Molecular crystals usually have low melting points.
D)Metallic crystals are usually good electrical conductors.
E)None of the statements is false.
A)Diamond is a covalent crystal.
B)The size of the unit cell of Li and Cs is the same.
C)Molecular crystals usually have low melting points.
D)Metallic crystals are usually good electrical conductors.
E)None of the statements is false.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following has the highest melting temperature?
A)H2O
B)CO2
C)S8
D)MgF2
E)P4
A)H2O
B)CO2
C)S8
D)MgF2
E)P4

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
71
A solid crystal of NaCl is
A)soft,low melting,a good electrical conductor
B)hard,high melting,a good electrical conductor
C)soft,low melting,a poor electrical conductor
D)hard,high melting,a poor electrical conductor
E)soft,high melting,a poor electrical conductor
A)soft,low melting,a good electrical conductor
B)hard,high melting,a good electrical conductor
C)soft,low melting,a poor electrical conductor
D)hard,high melting,a poor electrical conductor
E)soft,high melting,a poor electrical conductor

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
72
The unit cell in a certain lattice consists of a cube formed by an anion at each corner,an anion in the center,and a cation at the center of each face.The unit cell contains a net:
A)5 anions and 6 cations
B)5 anions and 3 cations
C)2 anions and 3 cations
D)3 anions and 4 cations
E)2 anions and 2 cations
A)5 anions and 6 cations
B)5 anions and 3 cations
C)2 anions and 3 cations
D)3 anions and 4 cations
E)2 anions and 2 cations

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
73
Lithium chloride crystallizes in a face-centered cubic structure.The unit cell length is 5.14 10-8 cm.The chloride ions are touching each other along the face diagonal of the unit cell.The lithium ions fit into the holes between the chloride ions.What is the mass of LiCl in a unit cell?
A)7.04 10-23 g
B)1.41 10-22 g
C)2.82 10-22 g
D)4.22 10-22 g
E)5.63 10-22 g
A)7.04 10-23 g
B)1.41 10-22 g
C)2.82 10-22 g
D)4.22 10-22 g
E)5.63 10-22 g

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
74
A certain solid substance that is very hard,has a high melting point,and is nonconducting unless melted is most likely to be:
A)I2
B)NaCl
C)CO2
D)H2O
E)Cu
A)I2
B)NaCl
C)CO2
D)H2O
E)Cu

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
75
On the basis of your knowledge of bonding in liquids and solids,which of the following has the lowest melting temperature?
A)NaCl
B)Na
C)Cl2
D)SiO2
E)More information is needed.
A)NaCl
B)Na
C)Cl2
D)SiO2
E)More information is needed.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following substances would you expect to have the lowest boiling point?
A)diamond
B)methane,CH4
C)sodium nitrate,NaNO3
D)glycerine,C3H5(OH)3
E)copper
A)diamond
B)methane,CH4
C)sodium nitrate,NaNO3
D)glycerine,C3H5(OH)3
E)copper

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
77
How would you expect the melting point of C[$X]4 to compare to that of [$I][$X]?
A)It should be lower because [$I][$X] forms an extended ionic crystal lattice,whereas C[$X]4 is a covalently-bonded small molecule.
B)It should be similar since they are both ionic solids.
C)It should be higher because the bonds are stronger due to the higher charge on C (+4).
D)It should be higher because C[$X]4 is larger than [$I][$X].
E)It is not possible to say anything about their melting points without more information.
A)It should be lower because [$I][$X] forms an extended ionic crystal lattice,whereas C[$X]4 is a covalently-bonded small molecule.
B)It should be similar since they are both ionic solids.
C)It should be higher because the bonds are stronger due to the higher charge on C (+4).
D)It should be higher because C[$X]4 is larger than [$I][$X].
E)It is not possible to say anything about their melting points without more information.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following is most likely to be a solid at room temperature?
A)Na2S
B)HF
C)NH3
D)N2
E)H2O
A)Na2S
B)HF
C)NH3
D)N2
E)H2O

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
79
An ionic crystalline solid,MX2,has a cubic unit cell.Which of the following arrangements of the ions is consistent with the stoichiometry of the compound?
A)M2+ ions at the corners and at each face,8 X- ions at the tetrahedral centers
B)M2+ ions at each face,X- ions at the corners
C)M2+ ions at the corners,X- ions at the faces,and 2 X- ions at the tetrahedralcenters
D)4 M2+ ions at the body centers,X- ions at the corners and at the faces
E)M2+ ions at the corners and at the faces,4 X- ions at the octahedral centers
A)M2+ ions at the corners and at each face,8 X- ions at the tetrahedral centers
B)M2+ ions at each face,X- ions at the corners
C)M2+ ions at the corners,X- ions at the faces,and 2 X- ions at the tetrahedralcenters
D)4 M2+ ions at the body centers,X- ions at the corners and at the faces
E)M2+ ions at the corners and at the faces,4 X- ions at the octahedral centers

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following statements about the closest packing of spheres in binary ionic solids is false?
A)The packing is done in a way that minimizes repulsions among ions with like charges.
B)The packing arrangement maximizes electrostatic attractions among oppositely charged ions.
C)For spheres of a given diameter,tetrahedral holes are larger than octahedral holes.
D)Trigonal holes are so small that they are never occupied.
E)None of these.
A)The packing is done in a way that minimizes repulsions among ions with like charges.
B)The packing arrangement maximizes electrostatic attractions among oppositely charged ions.
C)For spheres of a given diameter,tetrahedral holes are larger than octahedral holes.
D)Trigonal holes are so small that they are never occupied.
E)None of these.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck



