Deck 3: Stoichiometry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/134
Play
Full screen (f)
Deck 3: Stoichiometry
1
For which compound does 0.256 mole weigh 12.8 g?
A)C2H4O
B)CO2
C)CH3Cl
D)C2H6
E)none of these
A)C2H4O
B)CO2
C)CH3Cl
D)C2H6
E)none of these
CH3Cl
2
Naturally occurring element X exists in three isotopic forms: X-28 (27.977 amu,92.23% abundance),X-29 (28.976 amu,4.67% abundance),and X-30 (29.974 amu,3.10% abundance).Calculate the atomic weight of X.
A)28.09 amu
B)48.63 amu
C)27.16 amu
D)28.97 amu
E)86.93 amu
A)28.09 amu
B)48.63 amu
C)27.16 amu
D)28.97 amu
E)86.93 amu
28.09 amu
3
Calculate the molar mass of barium sulfite.
A)233.40 g/mol
B)201.40 g/mol
C)354.73 g/mol
D)514.87 g/mol
E)217.40 g/mol
A)233.40 g/mol
B)201.40 g/mol
C)354.73 g/mol
D)514.87 g/mol
E)217.40 g/mol
217.40 g/mol
4
The average mass of a carbon atom is 12.011.Assuming you were able to pick up only one carbon unit,the chances that you would randomly get one with a mass of 12.011 is
A)0%
B)0.011%
C)about 12%
D)12.011%
E)greater than 50%
A)0%
B)0.011%
C)about 12%
D)12.011%
E)greater than 50%

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
5
Naturally occurring element X exists in three isotopic forms: X-28 (27.977 amu,92.23% abundance),X-29 (28.976 amu,4.67% abundance),and X-30 (29.974 amu,3.10% abundance).What is the identity of element X?
A)Cu
B)Al
C)Ni
D)Si
E)Sr
A)Cu
B)Al
C)Ni
D)Si
E)Sr

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
6
The atomic mass of rhenium is 186.2.Given that 37.1% of natural rhenium is rhenium-185,what is the other stable isotope?
A)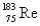
B)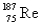
C)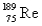
D)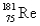
E)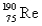
A)
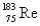
B)
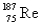
C)
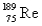
D)
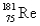
E)
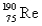

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
7
A sample of ammonia has a mass of 43.5 g.How many molecules are in this sample?
A)2.55 molecules
B)2.62 1025 molecules
C)2.36 1023 molecules
D)1.54 1024 molecules
E)8.63 10-16 molecules
A)2.55 molecules
B)2.62 1025 molecules
C)2.36 1023 molecules
D)1.54 1024 molecules
E)8.63 10-16 molecules

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
8
Iron is biologically important in the transport of oxygen by red blood cells from the lungs to the various organs of the body.In the blood of an adult human,there are approximately 2.64 1013 red blood cells with a total of 2.90 g of iron.On the average,how many iron atoms are present in each red blood cell? (molar mass Fe = 55.85 g/mol)
A)8.44 10-10
B)1.18 109
C)3.13 1022
D)2.64 1013
E)6.14 10-2
A)8.44 10-10
B)1.18 109
C)3.13 1022
D)2.64 1013
E)6.14 10-2

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
9
How many moles of hydrogen sulfide are contained in a 49.7-g sample of this gas?
A)0.686 mol
B)1.46 mol
C)83.8 mol
D)24.7 mol
E)2.92 mol
A)0.686 mol
B)1.46 mol
C)83.8 mol
D)24.7 mol
E)2.92 mol

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
10
A hypothetical element consists of two isotopes of masses 69.95 amu and 71.95 amu with abundances of 25.7% and 74.3%,respectively.What is the average atomic mass of this element?
A)70.95 amu
B)69.95 amu
C)70.5 amu
D)71.4 amu
E)71.95 amu
A)70.95 amu
B)69.95 amu
C)70.5 amu
D)71.4 amu
E)71.95 amu

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
11
What is the molar mass of ethanol (C2H5OH)?
A)45.06 g/mol
B)34.06 g/mol
C)46.07 g/mol
D)30.03 g/mol
E)105.03 g/mol
A)45.06 g/mol
B)34.06 g/mol
C)46.07 g/mol
D)30.03 g/mol
E)105.03 g/mol

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
12
Phosphoric acid can be prepared by reaction of sulfuric acid with "phosphate rock" according to the equation: Ca3(PO4)2 + 3H2SO4 3CaSO4 + 2H3PO4
How many oxygen atoms are there in 1.75 ng of Ca3(PO4)2?
A)3.40 1012
B)1.36 1013
C)8.43 1015
D)2.72 1022
E)2.72 1013
How many oxygen atoms are there in 1.75 ng of Ca3(PO4)2?
A)3.40 1012
B)1.36 1013
C)8.43 1015
D)2.72 1022
E)2.72 1013

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
13
Phosphoric acid can be prepared by reaction of sulfuric acid with "phosphate rock" according to the equation: Ca3(PO4)2 + 3H2SO4 3CaSO4 + 2H3PO4
What is the molar mass of Ca3(PO4)2?
A)310.18 g/mol
B)87.05 g/mol
C)278.18 g/mol
D)215.21 g/mol
E)166.02 g/mol
What is the molar mass of Ca3(PO4)2?
A)310.18 g/mol
B)87.05 g/mol
C)278.18 g/mol
D)215.21 g/mol
E)166.02 g/mol

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
14
Roundup,an herbicide manufactured by Monsanto,has the formula C3H8NO5P.How many moles of molecules are there in a 295.1-g sample of Roundup?
A)0.5729
B)2.137
C)1.745
D)16.39
E)none of these
A)0.5729
B)2.137
C)1.745
D)16.39
E)none of these

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
15
You have a sample of zinc (Zn)and a sample of aluminum (Al).You have an equal number of atoms in each sample.Which of the following statements concerning the masses of the samples is true?
A)The mass of the zinc sample is more than twice as great as the mass of the aluminum sample.
B)The mass of the zinc sample is more than the mass of the aluminum sample,but it is not twice as great.
C)The mass of the aluminum sample is more than twice as great as the mass of the zinc sample.
D)The mass of the aluminum sample is more than the mass of the zinc sample,but it is not twice as great.
E)The masses of each sample are equal.
A)The mass of the zinc sample is more than twice as great as the mass of the aluminum sample.
B)The mass of the zinc sample is more than the mass of the aluminum sample,but it is not twice as great.
C)The mass of the aluminum sample is more than twice as great as the mass of the zinc sample.
D)The mass of the aluminum sample is more than the mass of the zinc sample,but it is not twice as great.
E)The masses of each sample are equal.

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
16
Phosphorus has the molecular formula P4, and sulfur has the molecular formula S8.How many grams of phosphorus contain the same number of molecules as 4.23 g of sulfur?
A)2.04 g
B)0.490 g
C)4.08 g
D)4.23 g
E)none of these
A)2.04 g
B)0.490 g
C)4.08 g
D)4.23 g
E)none of these

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
17
A given sample of a xenon fluoride compound contains molecules of a single type XeFn,where n is some whole number.Given that 8.06 1020 molecules of XeFn weigh 0.227 g,calculate n.
A)1
B)6
C)4
D)3
E)2
A)1
B)6
C)4
D)3
E)2

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
18
Naturally occurring copper exists in two isotopic forms: 63Cu and 65Cu.The atomic mass of copper is 63.55 amu.What is the approximate natural abundance of 63Cu?
A)63%
B)90%
C)70%
D)50%
E)30%
A)63%
B)90%
C)70%
D)50%
E)30%

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
19
What is the mass of 4 atom(s)of copper in grams?
A)254.2 g
B)2.37 1021 g
C)9.57 10-24 g
D)6.022 1023 g
E)4.22 10-22 g
A)254.2 g
B)2.37 1021 g
C)9.57 10-24 g
D)6.022 1023 g
E)4.22 10-22 g

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
20
Consider the element indium,atomic number 49,atomic mass 114.8 g.The nucleus of an atom of indium-112 contains
A)49 protons,63 neutrons,49 electrons
B)49 protons,49 neutrons
C)49 protons,49 alpha particles
D)49 protons,63 neutrons
E)49 protons,112 neutrons
A)49 protons,63 neutrons,49 electrons
B)49 protons,49 neutrons
C)49 protons,49 alpha particles
D)49 protons,63 neutrons
E)49 protons,112 neutrons

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
21
A substance contains 35.0 g nitrogen,5.05 g hydrogen,and 60.0 g of oxygen.How many grams of hydrogen are there in a 153-g sample of this substance?
A)7.72 g
B)767 g
C)15.4 g
D)5.05 g
E)30.3 g
A)7.72 g
B)767 g
C)15.4 g
D)5.05 g
E)30.3 g

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
22
What is the mass of a 6.761-mol sample of sodium hydroxide?
A)40.00 g
B)270.4 g
C)162.3 g
D)5.916 g
E)0.1690 g
A)40.00 g
B)270.4 g
C)162.3 g
D)5.916 g
E)0.1690 g

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
23
The mineral vanadinite has the formula Pb5(VO4)3Cl.What mass percent of chlorine does it contain?
A)7.51 %
B)3.08 %
C)9.91 %
D)2.50 %
E)1.13 %
A)7.51 %
B)3.08 %
C)9.91 %
D)2.50 %
E)1.13 %

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
24
A substance,A2B,has the composition by mass of 60% A and 40% B.What is the composition of AB2 by mass?
A)40% A,60% B
B)50% A,50% B
C)27% A,73% B
D)33% A,67% B
E)none of these
A)40% A,60% B
B)50% A,50% B
C)27% A,73% B
D)33% A,67% B
E)none of these

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
25
One molecule of a compound weighs 2.93 10-22 g.The molar mass of this compound is:
A)2.06 g/mol
B)567 g/mol
C)168 g/mol
D)176 g/mol
E)none of these
A)2.06 g/mol
B)567 g/mol
C)168 g/mol
D)176 g/mol
E)none of these

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
26
Chlorous acid,HClO2,contains what percent hydrogen by mass?
A)1.92%
B)25.0%
C)23.4%
D)1.47%
E)5.18%
A)1.92%
B)25.0%
C)23.4%
D)1.47%
E)5.18%

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
27
NaHCO3 is the active ingredient in baking soda.How many grams of oxygen are in 0.52 g of NaHCO3?
A)0.099 g
B)0.019 g
C)6.19 103 g
D)0.033 g
E)0.30 g
A)0.099 g
B)0.019 g
C)6.19 103 g
D)0.033 g
E)0.30 g

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
28
The molar mass of an insecticide,dibromoethane,is 187.9 g/mol.Its molecular formula is C2H4Br2.What percent by mass of bromine does dibromoethane contain?
A)42.52%
B)2.14%
C)85.05%
D)12.78%
E)6.39%
A)42.52%
B)2.14%
C)85.05%
D)12.78%
E)6.39%

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
29
Each molecule of testosterone contains 19 atoms of carbon (plus other atoms).The mass percent of carbon in testosterone is 79.12%.What is the molar mass of testosterone?
A)576.8 g/mol
B)180.5 g/mol
C)228.2 g/mol
D)240.1 g/mol
E)288.4 g/mol
A)576.8 g/mol
B)180.5 g/mol
C)228.2 g/mol
D)240.1 g/mol
E)288.4 g/mol

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
30
A compound is composed of element X and hydrogen.Analysis shows the compound to be 80% X by mass,with three times as many hydrogen atoms as X atoms per molecule.Which element is element X?
A)He
B)C
C)F
D)S
E)none of these
A)He
B)C
C)F
D)S
E)none of these

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
31
A mixture of KCl and KNO3 is 44.20% potassium by mass.The percentage of KCl in the mixture is closest to
A)40%
B)50%
C)60%
D)70%
E)80%
A)40%
B)50%
C)60%
D)70%
E)80%

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
32
Hydrocortisone valerate is an ingredient in hydrocortisone cream,prescribed for skin problems.Its molecular formula is C26H38O6.What is the percent by mass of carbon in hydrocortisone valerate?
A)69.9%
B)43.6%
C)54.2%
D)76.9%
E)60.1%
A)69.9%
B)43.6%
C)54.2%
D)76.9%
E)60.1%

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
33
The molar mass of the compound formed by potassium and selenium is
A)157.2 g/mol
B)197.0 g/mol
C)118.1 g/mol
D)276.0 g/mol
E)196.3 g/mol
A)157.2 g/mol
B)197.0 g/mol
C)118.1 g/mol
D)276.0 g/mol
E)196.3 g/mol

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
34
A substance contains 23.0 g sodium,27.0 g aluminum,and 114 g fluorine.How many grams of sodium are there in a 102-g sample of the substance?
A)4.43 g
B)23.0 g
C)14.3 g
D)102 g
E)none of these
A)4.43 g
B)23.0 g
C)14.3 g
D)102 g
E)none of these

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
35
Which compound contains the highest percent by mass of hydrogen?
A)HCl
B)H2O
C)H2SO4
D)H2S
E)HF
A)HCl
B)H2O
C)H2SO4
D)H2S
E)HF

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following compounds has the same percent composition by mass as styrene,C8H8?
A)acetylene,C2H2
B)benzene,C6H6
C)cyclobutadiene,C4H4
D)" -ethyl naphthalene",C12H12
E)all of these
A)acetylene,C2H2
B)benzene,C6H6
C)cyclobutadiene,C4H4
D)" -ethyl naphthalene",C12H12
E)all of these

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
37
How many atoms of hydrogen are present in 7.63 g of ammonia?
A)2.70 1023
B)1.52 1024
C)1.38 1025
D)8.09 1023
E)1.12 1020
A)2.70 1023
B)1.52 1024
C)1.38 1025
D)8.09 1023
E)1.12 1020

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
38
What is the molar mass of cryolite (Na3AlF6)?
A)209.9 g/mol
B)185.3 g/mol
C)210.0 g/mol
D)104.2 g/mol
E)68.97 g/mol
A)209.9 g/mol
B)185.3 g/mol
C)210.0 g/mol
D)104.2 g/mol
E)68.97 g/mol

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
39
How many grams of potassium are in 27.8 g of K2CrO7?
A)4.49 g
B)1.422 g
C)8.98 g
D)78.2 g
E)55.6 g
A)4.49 g
B)1.422 g
C)8.98 g
D)78.2 g
E)55.6 g

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
40
An oxide of iron has the formula Fe3O4.What mass percent of iron does it contain?
A)0.72%
B)28%
C)30%
D)70%
E)72%
A)0.72%
B)28%
C)30%
D)70%
E)72%

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
41
A 2.80-g sample of an oxide of bromine is converted to 4.698 g of AgBr.Calculate the empirical formula of the oxide.(molar mass for AgBr = 187.78 g/mol)
A)BrO3
B)BrO2
C)BrO
D)Br2O
E)none of these
A)BrO3
B)BrO2
C)BrO
D)Br2O
E)none of these

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
42
Vitamin C contains the elements C,H,and O.It is known to contain 40.9% C and 4.58% H by mass.The molar mass of vitamin C has been found to be about 180 g/mol.The molecular formula for vitamin C is:
A)C2H3O2
B)C3H4O3
C)C4H6O4
D)C6H8O6
E)C14H18
A)C2H3O2
B)C3H4O3
C)C4H6O4
D)C6H8O6
E)C14H18

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
43
A hydrocarbon (a compound consisting solely of carbon and hydrogen)is found to be 85.6% carbon by mass.What is the empirical formula for this compound?
A)CH
B)CH2
C)C2H
D)C3H
E)CH4
A)CH
B)CH2
C)C2H
D)C3H
E)CH4

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
44
The empirical formula of styrene is CH;its molar mass is 104.1 g/mol.What is the molecular formula of styrene?
A)C2H4
B)C8H8
C)C10H12
D)C6H6
E)none of these
A)C2H4
B)C8H8
C)C10H12
D)C6H6
E)none of these

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
45
You take an aspirin tablet (a compound consisting solely of carbon,hydrogen,and oxygen)with a mass of 1.00 g,burn it in air,and collect 2.20 g of carbon dioxide and 0.400 g water.The molar mass of aspirin is between 170 and 190 g/mol.The molecular formula of aspirin is
A)C6H8O5
B)C9H8O4
C)C8H10O5
D)C10H6O4
E)none of these
A)C6H8O5
B)C9H8O4
C)C8H10O5
D)C10H6O4
E)none of these

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
46
Ammonium chromate, (NH4)2CrO4,contains what percent nitrogen by mass?
A)36.8%
B)9.2%
C)18.4%
D)11.9%
E)none of these
A)36.8%
B)9.2%
C)18.4%
D)11.9%
E)none of these

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following equations is not balanced?
A)4Al + 3O2 2Al2O3
B)C2H6 +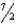 O2 2CO2 + 3H2O
O2 2CO2 + 3H2O
C)2KClO3 2KCl +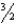 O2
O2
D)4P4 + 5S8 4P4S10
E)P4 + 5O2 P4O10
A)4Al + 3O2 2Al2O3
B)C2H6 +
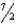 O2 2CO2 + 3H2O
O2 2CO2 + 3H2OC)2KClO3 2KCl +
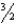 O2
O2D)4P4 + 5S8 4P4S10
E)P4 + 5O2 P4O10

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
48
What is the coefficient for water when the following equation is balanced? As(OH)3(s)+ H2SO4(aq) As2(SO4)3(aq)+ H2O(l)
A)1
B)2
C)4
D)6
E)12
A)1
B)2
C)4
D)6
E)12

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
49
The empirical formula of a group of compounds is CHCl.Lindane,a powerful insecticide,is a member of this group.The molar mass of lindane is 290.8 g/mol.How many atoms of carbon does a molecule of lindane contain?
A)2
B)3
C)4
D)6
E)8
A)2
B)3
C)4
D)6
E)8

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
50
Adipic acid contains 49.32% C,43.84% O,and 6.85% H by mass.What is the empirical formula?
A)C3H5O2
B)C3H3O4
C)C2HO3
D)C2H5O4
E)C3HO3
A)C3H5O2
B)C3H3O4
C)C2HO3
D)C2H5O4
E)C3HO3

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
51
You heat 3.854 g of a mixture of Fe3O4 and FeO to form 4.148 g Fe2O3.The mass percent of FeO originally in the mixture was:
A)92.9%
B)55.8%
C)44.2%
D)38.5%
E)none of these
A)92.9%
B)55.8%
C)44.2%
D)38.5%
E)none of these

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
52
In balancing an equation,we change the __________ to make the number of atoms on each side of the equation balance.
A)formulas of compounds in the reactants
B)coefficients of compounds
C)formulas of compounds in the products
D)subscripts of compounds
E)none of these
A)formulas of compounds in the reactants
B)coefficients of compounds
C)formulas of compounds in the products
D)subscripts of compounds
E)none of these

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
53
Balanced chemical equations imply which of the following?
A)Numbers of molecules are conserved in chemical change.
B)Numbers of atoms are conserved in chemical change.
C)Volume is conserved in chemical change.
D)A and B
E)B and C
A)Numbers of molecules are conserved in chemical change.
B)Numbers of atoms are conserved in chemical change.
C)Volume is conserved in chemical change.
D)A and B
E)B and C

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
54
A chloride of rhenium contains 63.6% rhenium.What is the formula of this compound?
A)ReCl
B)ReCl3
C)ReCl5
D)ReCl7
E)Re2Cl3
A)ReCl
B)ReCl3
C)ReCl5
D)ReCl7
E)Re2Cl3

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
55
A 0.4987-g sample of a compound known to contain only carbon,hydrogen,and oxygen was burned in oxygen to yield 0.9267 g of CO2 and 0.1897 g of H2O.What is the empirical formula of the compound?
A)CHO
B)C2H2O
C)C3H3O2
D)C6H3O2
E)C3H6O2
A)CHO
B)C2H2O
C)C3H3O2
D)C6H3O2
E)C3H6O2

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
56
A compound composed of sulfur and fluorine is found to contain 62.79% by mass of sulfur.If the molar mass of the compound is 102.13 g/mol,what is its molecular formula?
A)S3F
B)S2F4
C)SF
D)S2F
E)S2F2
A)S3F
B)S2F4
C)SF
D)S2F
E)S2F2

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
57
The molecular formula always represents the total number of atoms of each element present in a compound.

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
58
Suppose you are given the percent by mass of the elements in a compound and you wish to determine the empirical formula.Which of the following is true?
A)You must convert percent by mass to relative numbers of atoms.
B)You must assume exactly 100.0 g of the compound.
C)You must divide all of the percent by mass numbers by the smallest percent by mass.
D)You cannot solve for the empirical formula without the molar mass.
E)At least two of the above (A-D)are true.
A)You must convert percent by mass to relative numbers of atoms.
B)You must assume exactly 100.0 g of the compound.
C)You must divide all of the percent by mass numbers by the smallest percent by mass.
D)You cannot solve for the empirical formula without the molar mass.
E)At least two of the above (A-D)are true.

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
59
How many of the following are true concerning balanced chemical equations?
I.The number of molecules is conserved.
II.The coefficients for the reactants tell you how much of each reactant you are given.
III.Atoms are neither created nor destroyed.
IV.The coefficients indicate the mass ratios of the substances used.
V.The sum of the coefficients on the reactant side equals the sum of the coefficients on
The product side.
A)1
B)2
C)3
D)4
E)5
I.The number of molecules is conserved.
II.The coefficients for the reactants tell you how much of each reactant you are given.
III.Atoms are neither created nor destroyed.
IV.The coefficients indicate the mass ratios of the substances used.
V.The sum of the coefficients on the reactant side equals the sum of the coefficients on
The product side.
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
60
Oxides of copper include CuO and Cu2O.You heat 1.51 g of one of these copper oxides in the absence of air and obtain 1.21 g of Cu.
True or false: You must have had CuO.
True or false: You must have had CuO.

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
61
What is the coefficient for oxygen when the following equation is balanced? NH3(g)+ O2(g) NO2(g)+ H2O(g)
A)3
B)6
C)7
D)12
E)14
A)3
B)6
C)7
D)12
E)14

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
62
When the equation C6H14 + O2 CO2 + H2O is balanced with the smallest set of integers,the sum of the coefficients is
A)4
B)47
C)15
D)27
E)34
A)4
B)47
C)15
D)27
E)34

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
63
Indium reacts with chlorine to form InCl3.In the balanced equation for this reaction,the coefficient of the indium trichloride is
A)1
B)2
C)3
D)4
E)6
A)1
B)2
C)3
D)4
E)6

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
64
wPCl5 + xH2O yPOCl3 + zHCl The above equation is properly balanced when:
A)w = 1,x = 2,y = 2,z = 4
B)w = 2,x = 2,y = 2,z = 2
C)w = 2,x = 2,y = 2,z = 1
D)w = 1,x = 1,y = 1,z = 2
E)none of these
A)w = 1,x = 2,y = 2,z = 4
B)w = 2,x = 2,y = 2,z = 2
C)w = 2,x = 2,y = 2,z = 1
D)w = 1,x = 1,y = 1,z = 2
E)none of these

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
65
To balance a chemical equation,the coefficients must not be changed.

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
66
What is the sum of the coefficients of the following equation when it is balanced using smallest whole numbers? NaNH2 + NaNO3 NaN3 +NaOH + NH3
A)5
B)6
C)7
D)8
E)9
A)5
B)6
C)7
D)8
E)9

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
67
The correct coefficients to balance the following reaction are,in order: I2O5 + H2S I2 + SO2 + H2O
A)1,1,1,1,1
B)1,2,1,2,1
C)2,4,1,4,5
D)2,5,2,5,5
E)3,5,3,5,5
A)1,1,1,1,1
B)1,2,1,2,1
C)2,4,1,4,5
D)2,5,2,5,5
E)3,5,3,5,5

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
68
You heat 3.869 g of a mixture of Fe3O4 and FeO to form 4.141 g Fe2O3.The mass of oxygen reacted is
A)0.272 g
B)0.476 g
C)1.242 g
D)1.000 g
E)none of these
A)0.272 g
B)0.476 g
C)1.242 g
D)1.000 g
E)none of these

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
69
The Claus reactions,shown below,are used to generate elemental sulfur from hydrogen sulfide. 2H2S + 3O2 2SO2 + 2H2O
SO2 + 2H2S 3S + 2H2O
How much sulfur (in grams)is produced from 21.5 grams of O2?
A)97.0 g
B)14.4 g
C)21.5 g
D)43.1 g
E)none of these
SO2 + 2H2S 3S + 2H2O
How much sulfur (in grams)is produced from 21.5 grams of O2?
A)97.0 g
B)14.4 g
C)21.5 g
D)43.1 g
E)none of these

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following equations correctly describes the combustion of CH4 and O2 to produce water (H2O)and carbon dioxide (CO2)?
A)CH4 +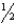 O2 CO2 + H2O
O2 CO2 + H2O
B)CH4 + O2 CO2 + 2H2O
C)CH4 + 2O2 CO2 + 2H2O
D)CH4 + 3O2 2CO2 + H2O
E)2CH4 + 3O2 2CO2 + 2H2O
A)CH4 +
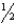 O2 CO2 + H2O
O2 CO2 + H2OB)CH4 + O2 CO2 + 2H2O
C)CH4 + 2O2 CO2 + 2H2O
D)CH4 + 3O2 2CO2 + H2O
E)2CH4 + 3O2 2CO2 + 2H2O

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
71
When the following equation is balanced,what is the sum of the coefficients? Al2(SO4)3 + Ca(OH)2 Al(OH)3 + CaSO4
A)4
B)9
C)8
D)7
E)10
A)4
B)9
C)8
D)7
E)10

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
72
Consider a specific chemical reaction represented by the equation aA + bB cC + dD.In this equation the letters A,B,C,and D represent chemicals,and the letters a,b,c,and d represent coefficients in the balanced equation.
-How many possible values are there for the quantity "c"?
A)1
B)2
C)3
D)4
E)infinite
-How many possible values are there for the quantity "c"?
A)1
B)2
C)3
D)4
E)infinite

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
73
Consider a specific chemical reaction represented by the equation aA + bB cC + dD.In this equation the letters A,B,C,and D represent chemicals,and the letters a,b,c,and d represent coefficients in the balanced equation.
-How many possible values are there for the quantity "c/d"?
A)1
B)2
C)3
D)4
E)infinite
-How many possible values are there for the quantity "c/d"?
A)1
B)2
C)3
D)4
E)infinite

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
74
In the balanced equation for the reaction: xP4O6(s)+ yH2O(l) zH3PO3(aq)
If x equals 2,the coefficient z equals:
A)2
B)4
C)6
D)10
E)none of these
If x equals 2,the coefficient z equals:
A)2
B)4
C)6
D)10
E)none of these

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
75
When balancing a chemical equation,it is generally best to start with the least complicated molecules.

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
76
How many of the following statements are true concerning chemical equations? I.Coefficients can be fractions.
II.Subscripts can be fractions.
III.Coefficients represent the relative masses of the reactants and/or products.
IV.Changing the subscripts to balance an equation can only be done once.
V.Atoms are conserved when balancing chemical equations.
A)1
B)2
C)3
D)4
E)5
II.Subscripts can be fractions.
III.Coefficients represent the relative masses of the reactants and/or products.
IV.Changing the subscripts to balance an equation can only be done once.
V.Atoms are conserved when balancing chemical equations.
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
77
A reaction occurs between sodium carbonate and hydrochloric acid producing sodium chloride,carbon dioxide,and water.The correct set of coefficients,respectively,for the balanced reaction is:
A)3 6 6 3 4
B)8 6 5 10 5
C)5 10 10 5 5
D)1 2 2 1 1
E)none of these
A)3 6 6 3 4
B)8 6 5 10 5
C)5 10 10 5 5
D)1 2 2 1 1
E)none of these

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
78
Potassium forms an oxide containing 1 oxygen atom for every 2 atoms of potassium.What is the coefficient of oxygen in the balanced equation for the reaction of potassium with oxygen to form this oxide?
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
79
Determine the coefficient for O2 when the following equation is balanced in standard form (smallest whole numbers). C8H18(g)+ O2(g) CO2(g)+ H2O(g)
A)8
B)17
C)18
D)25
E)16
A)8
B)17
C)18
D)25
E)16

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
80
Give (in order)the correct coefficients to balance the following reaction: H2SnCl6 + H2S SnS2 + HCl
A)1,2,1,6
B)1,2,2,2
C)1,1,1,6
D)6,2,1,1
E)2,4,2,6
A)1,2,1,6
B)1,2,2,2
C)1,1,1,6
D)6,2,1,1
E)2,4,2,6

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck



