Deck 3: Alkenes and Alkynes: the Nature of Organic Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Match between columns
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/60
Play
Full screen (f)
Deck 3: Alkenes and Alkynes: the Nature of Organic Reactions
1
Instructions: Pent-2-ene is an example of a disubstituted alkene. Use this alkene to answer the following question(s).
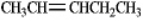 Refer to instructions. Circle the isomer of pent-2-ene that is most stable.
Refer to instructions. Circle the isomer of pent-2-ene that is most stable.
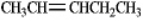 Refer to instructions. Circle the isomer of pent-2-ene that is most stable.
Refer to instructions. Circle the isomer of pent-2-ene that is most stable.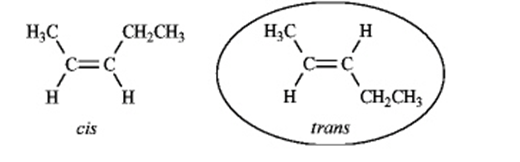
2
Instructions: In the reaction below:
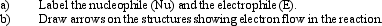
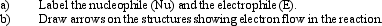
Label and indicate flow: 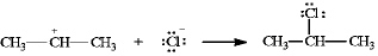
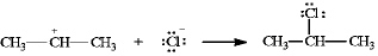
3
Instructions: Provide names for each structure below. Be sure to include the cis,trans or E,Z designations where applicable.
Name and designate: 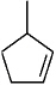

4
Instructions: Provide names for each structure below. Be sure to include the cis,trans or E,Z designations where applicable.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
5
Instructions: Consider the reaction of 2-bromo-2-methylpropane with water, shown below, to answer the following question(s).
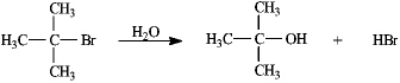 Refer to instructions. This reaction is an example of:
Refer to instructions. This reaction is an example of:
A) a substitution reaction.
B) a rearrangement reaction.
C) an elimination reaction.
D) an addition reaction.
 Refer to instructions. This reaction is an example of:
Refer to instructions. This reaction is an example of:A) a substitution reaction.
B) a rearrangement reaction.
C) an elimination reaction.
D) an addition reaction.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
6
The following group is a substituent on a molecule. What is an accepted IUPAC name for this group?
A) propenyl
B) allyl
C) vinyl
D) propylene
E) either a or b
A) propenyl
B) allyl
C) vinyl
D) propylene
E) either a or b

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following substituents has the highest priority according to the Cahn-Ingold-Prelog system?
A) -NH2
B) -NHCH3
C) -CH2NH2
D) -CH2NHCH3
A) -NH2
B) -NHCH3
C) -CH2NH2
D) -CH2NHCH3

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
8
What is the IUPAC name of the following compound?
A. (E)-3-methylpent-3-ene
B. (Z)-3-methylpent-3-ene
C. (E)-3-methylpent-2-ene
D. (Z)-3-methylpent-2-ene
A. (E)-3-methylpent-3-ene
B. (Z)-3-methylpent-3-ene
C. (E)-3-methylpent-2-ene
D. (Z)-3-methylpent-2-ene

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
9
Instructions: Assign E or Z configurations to each alkene below.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
10
Instructions: Draw structures corresponding to each name below.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
11
Instructions: Draw structures corresponding to each name below.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following substituents has the highest priority according to the Cahn-Ingold-Prelog system?
A) -COOH
B) -CHO
C) -CH2OH
D) -CH3
A) -COOH
B) -CHO
C) -CH2OH
D) -CH3

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
13
Rank each set of substituents using the Cahn-Ingold-Prelog sequence rules by numbering the highest priority substituent 1 and numbering the lowest priority substituent 4. Place the number in the blank below the substituent.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
14
Choose substituents X and Y (listed in order below) for the following compound so as to make a Z isomer.
A. -Br, -NHCH3
B. -F, -CHO
C. -I, -OCH3
D. -COOH, -CH2NH2
E. -Br, -COOH
A. -Br, -NHCH3
B. -F, -CHO
C. -I, -OCH3
D. -COOH, -CH2NH2
E. -Br, -COOH

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
15
Instructions: Use the reaction energy diagram below to answer the following question(s).

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
16
Instructions: Provide names for each structure below. Be sure to include the cis,trans or E,Z designations where applicable.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
17
Name this compound.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
18
Instructions: Match each definition to one of the terms below.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
19
Instructions: Pent-2-ene is an example of a disubstituted alkene. Use this alkene to answer the following question(s).
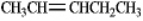 Refer to instructions. Draw the cis and trans isomers of pent-2-ene and label them.
Refer to instructions. Draw the cis and trans isomers of pent-2-ene and label them.
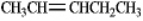 Refer to instructions. Draw the cis and trans isomers of pent-2-ene and label them.
Refer to instructions. Draw the cis and trans isomers of pent-2-ene and label them.
Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
20
Instructions: Match each definition to one of the terms below.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
21
The structures below show the stepwise bond making and bond breaking in this reaction. Draw curved arrows to show the electron flow that has occurred in each step.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
22
Instructions: Identify and label the nucleophile and electrophile in each reaction below.
Identify and label: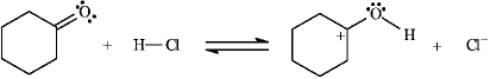
Identify and label:
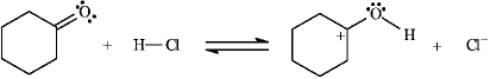

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
23
Instructions: Classify each structure below as a nucleophile or electrophile, and briefly explain your choice.
Classify and explain:

Classify and explain:


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
24
Instructions: The reaction below is commonly used as a laboratory preparation of cyclohexene. Use this reaction to answer the following question(s). 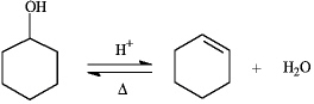 Refer to instructions. The forward and reverse reactions are classified, respectively, as:
Refer to instructions. The forward and reverse reactions are classified, respectively, as:
A) addition, elimination
B) elimination, substitution
C) elimination, addition
D) elimination, rearrangement
E) substitution, addition
 Refer to instructions. The forward and reverse reactions are classified, respectively, as:
Refer to instructions. The forward and reverse reactions are classified, respectively, as:A) addition, elimination
B) elimination, substitution
C) elimination, addition
D) elimination, rearrangement
E) substitution, addition

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
25
Instructions: Match each definition to one of the terms below.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
25
Instructions: Identify the functional groups present in each compound below, and predict the direction of polarity in each.
Identify and predict:
mustard gas Cl-CH2CH2-S-CH2CH2-Cl
Identify and predict:
mustard gas Cl-CH2CH2-S-CH2CH2-Cl

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
26
Predict the product of the following reaction of Prostaglandin H2 by interpreting the flow of electrons as indicated by the curved arrows.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
27
Instructions: Identify and label the nucleophile and electrophile in each reaction below.
Identify and label:
Identify and label:


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
28
Instructions: Identify the functional groups present in each compound below, and predict the direction of polarity in each.
Identify and predict:
Identify and predict:


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following is a characteristic of a polar reaction?
A) symmetrical bond making and breaking
B) one electron from each reactant forms the bond
C) involves a neutral species with an unpaired electron
D) are more common that radical reactions
E) all of these
A) symmetrical bond making and breaking
B) one electron from each reactant forms the bond
C) involves a neutral species with an unpaired electron
D) are more common that radical reactions
E) all of these

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
30
Instructions: In the reaction below:
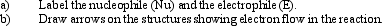 Label and indicate flow:
Label and indicate flow: 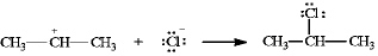
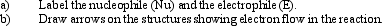 Label and indicate flow:
Label and indicate flow: 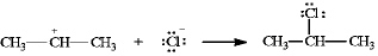

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
30
In an organic reaction, which of the following is most likely to function as only a nucleophile?
A) BF3
B) (CH3)2CH2NH2
C) Fe2+
D) CH3CH2S-
E) both a and c
A) BF3
B) (CH3)2CH2NH2
C) Fe2+
D) CH3CH2S-
E) both a and c

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
31
Instructions: Add curved arrows to the following reaction(s) to indicate the flow of electrons in each.
Indicate flow: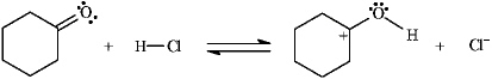
Indicate flow:
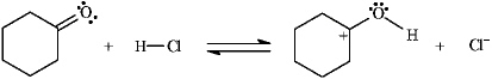

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
32
Instructions: Assign E or Z configurations to each alkene below.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
33
Instructions: Match each definition to one of the terms below.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
33
Below are all the chemical structures and intermediates involved in a reaction. On the structures provided, show all electron flow using the arrow formalism for the complete stepwise mechanism.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
34
Instructions: Add curved arrows to the following reaction(s) to indicate the flow of electrons in each.
Indicate flow: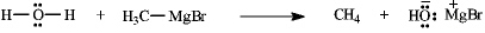
Indicate flow:
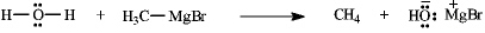

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
35
Instructions: Classify each reaction below as a(n):

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
36
Instructions: Consider the reaction of 2-bromo-2-methylpropane with water, shown below, to answer the following question(s). 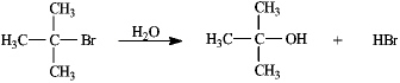 Refer to instructions. This reaction is an example of:
Refer to instructions. This reaction is an example of:
A) a substitution reaction.
B) a rearrangement reaction.
C) an elimination reaction.
D) an addition reaction.
 Refer to instructions. This reaction is an example of:
Refer to instructions. This reaction is an example of:A) a substitution reaction.
B) a rearrangement reaction.
C) an elimination reaction.
D) an addition reaction.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
37
Match between columns

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
38
Instructions: Classify each structure below as a nucleophile or electrophile, and briefly explain your choice.
Classify and explain:
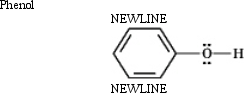
Classify and explain:


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
41
Instructions: Draw structures corresponding to each name below.
Draw:
trans-4,4-dimethylpent-2-ene
Draw:
trans-4,4-dimethylpent-2-ene

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
42
Name this compound. 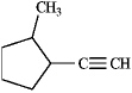


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
43
Instructions: Provide names for each structure below. Be sure to include the cis,trans or E,Z designations where applicable.
Name and designate::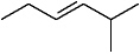
Name and designate::


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
44
Instructions: Draw structures corresponding to each name below.
Draw:
(3E)-3,7-dimethylocta-1,3,6-triene
Draw:
(3E)-3,7-dimethylocta-1,3,6-triene

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
45
Choose substituents X and Y (listed in order below) for the following compound so as to make a Z isomer. 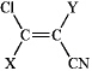
A) -Br, -NHCH3
B) -F, -CHO
C) -I, -OCH3
D) -COOH, -CH2NH2
E) -Br, -COOH

A) -Br, -NHCH3
B) -F, -CHO
C) -I, -OCH3
D) -COOH, -CH2NH2
E) -Br, -COOH

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
46
Instructions: Assign E or Z configurations to each alkene below.
Assign: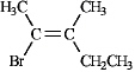
Assign:


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
47
Rank each set of substituents using the Cahn-Ingold-Prelog sequence rules by numbering the highest priority substituent 1 and numbering the lowest priority substituent 4. Place the number in the blank below the substituent. 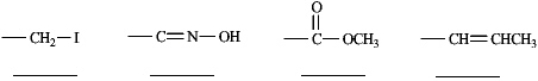
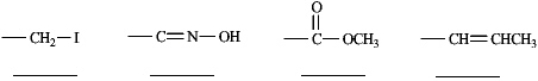

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
48
Instructions: Pent-2-ene is an example of a disubstituted alkene. Use this alkene to answer the following question(s). 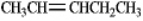 Refer to instructions. Circle the isomer of pent-2-ene that is most stable.
Refer to instructions. Circle the isomer of pent-2-ene that is most stable.
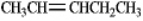 Refer to instructions. Circle the isomer of pent-2-ene that is most stable.
Refer to instructions. Circle the isomer of pent-2-ene that is most stable.
Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
49
Instructions: Provide names for each structure below. Be sure to include the cis,trans or E,Z designations where applicable.
Name and designate: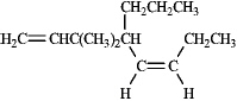
Name and designate:


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following substituents has the highest priority according to the Cahn-Ingold-Prelog system?
A) -NH2
B) -NHCH3
C) -CH2NH2
D) -CH2NHCH3
A) -NH2
B) -NHCH3
C) -CH2NH2
D) -CH2NHCH3

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
51
Predict the product of the following reaction of Prostaglandin H2 by interpreting the flow of electrons as indicated by the curved arrows. 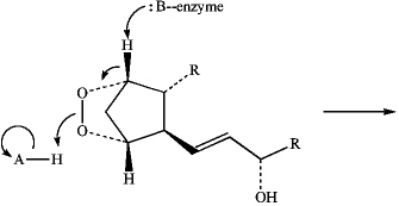


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following substituents has the highest priority according to the Cahn-Ingold-Prelog system?
A) -COOH
B) -CHO
C) -CH2OH
D) -CH3
A) -COOH
B) -CHO
C) -CH2OH
D) -CH3

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
53
Below are all the chemical structures and intermediates involved in a reaction. On the structures provided, show all electron flow using the arrow formalism for the complete stepwise mechanism.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
54
Instructions: Provide names for each structure below. Be sure to include the cis,trans or E,Z designations where applicable.
Name and designate: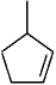
Name and designate:


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
55
What is the IUPAC name of the following compound? 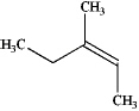
A) (E)-3-methylpent-3-ene
B) (Z)-3-methylpent-3-ene
C) (E)-3-methylpent-2-ene
D) (Z)-3-methylpent-2-ene

A) (E)-3-methylpent-3-ene
B) (Z)-3-methylpent-3-ene
C) (E)-3-methylpent-2-ene
D) (Z)-3-methylpent-2-ene

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
56
Instructions: Pent-2-ene is an example of a disubstituted alkene. Use this alkene to answer the following question(s). 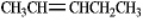 Refer to instructions. Draw the cis and trans isomers of pent-2-ene and label them.
Refer to instructions. Draw the cis and trans isomers of pent-2-ene and label them.
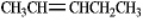 Refer to instructions. Draw the cis and trans isomers of pent-2-ene and label them.
Refer to instructions. Draw the cis and trans isomers of pent-2-ene and label them.
Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
57
Instructions: Assign E or Z configurations to each alkene below.
Assign: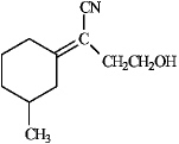
Assign:


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
58
The following group is a substituent on a molecule. What is an accepted IUPAC name for this group? 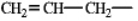
A) propenyl
B) allyl
C) vinyl
D) propylene
E) either a or b
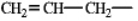
A) propenyl
B) allyl
C) vinyl
D) propylene
E) either a or b

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
59
The structures below show the stepwise bond making and bond breaking in this reaction. Draw curved arrows to show the electron flow that has occurred in each step. 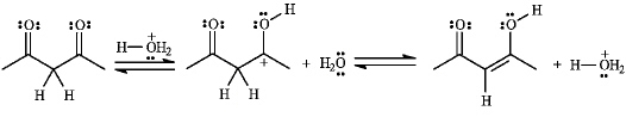


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck



