Deck 8: Chemical Bonding and Climate Change
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/104
Play
Full screen (f)
Deck 8: Chemical Bonding and Climate Change
1
Which of the following is the correct Lewis symbol for the oxide anion (O2-)? 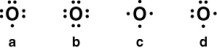
A) a
B) b
C) c
D) d
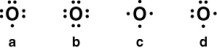
A) a
B) b
C) c
D) d
b
2
Which of the following has the most valence electrons?
A) HF
B) NS
C) CN-
D) ArF
E) ArF+
A) HF
B) NS
C) CN-
D) ArF
E) ArF+
ArF
3
Which of the following ions does not have a complete valence-shell octet?
A) B3+
B) I-
C) Ca2+
D) Pb2+
E) S2-
A) B3+
B) I-
C) Ca2+
D) Pb2+
E) S2-
Pb2+
4
Indicate which of the following molecules has only a single bond.
A) O2
B) CO
C) CN-
D) N2
E) Cl2
A) O2
B) CO
C) CN-
D) N2
E) Cl2

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
5
How many valence electrons does S2- have?
A) 8
B) 6
C) 16
D) 4
E) 14
A) 8
B) 6
C) 16
D) 4
E) 14

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
6
How many valence electrons are there in HCl?
A) 8
B) 7
C) 6
D) 2
E) 4
A) 8
B) 7
C) 6
D) 2
E) 4

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
7
Which one of the following Lewis symbols for these atoms is correct? 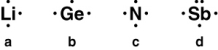
A) a
B) b
C) c
D) d
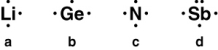
A) a
B) b
C) c
D) d

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
8
A covalent bond results when __________
A) electrons are transferred from one atom to another atom.
B) atoms pool their electrons to form a "sea" of electrons.
C) atoms have outer electrons with the same principal quantum number.
D) electrons are shared between a pair of atoms.
E) an atom has 8 valence electrons.
A) electrons are transferred from one atom to another atom.
B) atoms pool their electrons to form a "sea" of electrons.
C) atoms have outer electrons with the same principal quantum number.
D) electrons are shared between a pair of atoms.
E) an atom has 8 valence electrons.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
9
Indicate which of the following molecules has four shared electrons.
A) O2
B) CO
C) HI
D) N2
E) Cl2
A) O2
B) CO
C) HI
D) N2
E) Cl2

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
10
How many valence electrons does aluminum have?
A) 1
B) 2
C) 3
D) 11
E) 13
A) 1
B) 2
C) 3
D) 11
E) 13

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
11
Indicate the ion that has 8 valence electrons.
A) H-
B) Na+
C) Al3+
D) P3-
E) Ar
A) H-
B) Na+
C) Al3+
D) P3-
E) Ar

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
12
How many valence electrons are there in BrO-?
A) 7
B) 6
C) 13
D) 14
E) 18
A) 7
B) 6
C) 13
D) 14
E) 18

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
13
How many valence electrons are contained in CO+?
A) 10
B) 11
C) 9
D) 8
E) 14
A) 10
B) 11
C) 9
D) 8
E) 14

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
14
Indicate which molecule contains the largest number of nonbonding electrons.
A) H2
B) CO
C) N2
D) NO
E) O2
A) H2
B) CO
C) N2
D) NO
E) O2

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
15
Which type of bonding involves the sharing of valence electrons by two atoms?
A) covalent
B) ionic
C) polar ionic
D) intramolecular
E) metallic
A) covalent
B) ionic
C) polar ionic
D) intramolecular
E) metallic

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following has 13 valence electrons?
A) SH-
B) IO
C) NO
D) ICl
E) Cl2
A) SH-
B) IO
C) NO
D) ICl
E) Cl2

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following elements has 6 valence electrons?
A) C
B) S
C) N
D) Br
E) Be
A) C
B) S
C) N
D) Br
E) Be

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
18
How many shared electrons are there in the Lewis structure of CN-?
A) 3
B) 6
C) 4
D) 2
E) 10
A) 3
B) 6
C) 4
D) 2
E) 10

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following molecular ions has 3 valence electrons?
A) H2+
B) He2+
C) C2+
D) B2+
E) O2-
A) H2+
B) He2+
C) C2+
D) B2+
E) O2-

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
20
How many shared electron pairs are there in the Lewis structure of CN-?
A) 3
B) 6
C) 4
D) 2
E) 10
A) 3
B) 6
C) 4
D) 2
E) 10

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following are listed in order of decreasing electronegativity?
A) F > S > Na > H
B) F > N > P > O
C) N > P > Si > S
D) F > Cl > Br > C
E) Te > Se > S > O
A) F > S > Na > H
B) F > N > P > O
C) N > P > Si > S
D) F > Cl > Br > C
E) Te > Se > S > O

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
22
Electronegativity increases from left to right across a period because of __________
A) increased shielding of valence electrons by the core electrons.
B) an increase in the number of protons in the nucleus.
C) a decrease in the effective nuclear charge (Zeff).
D) an increase in the shielding of core electrons by the valence electrons.
E) a decrease in the ionization potential.
A) increased shielding of valence electrons by the core electrons.
B) an increase in the number of protons in the nucleus.
C) a decrease in the effective nuclear charge (Zeff).
D) an increase in the shielding of core electrons by the valence electrons.
E) a decrease in the ionization potential.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
23
In general, resonance __________ electrons and __________ molecules.
A) delocalizes, stabilizes
B) destabilizes, destabilizes
C) localizes, stabilizes
D) delocalizes, destabilizes
E) Localizes, stabilizes
A) delocalizes, stabilizes
B) destabilizes, destabilizes
C) localizes, stabilizes
D) delocalizes, destabilizes
E) Localizes, stabilizes

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
24
In which bond does the Cl atom have the highest electron density?
A) H-Cl
B) O -Cl
C) S -Cl
D) N-Cl
E) Br -Cl
A) H-Cl
B) O -Cl
C) S -Cl
D) N-Cl
E) Br -Cl

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
25
How many covalent bonds are there in SH-?
A) 1
B) 2
C) 3
D) 0
E) 4
A) 1
B) 2
C) 3
D) 0
E) 4

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following bonds is least polar?
A) H - O
B) H - N
C) H - B
D) H - C
E) H - F
A) H - O
B) H - N
C) H - B
D) H - C
E) H - F

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
27
Resonance structures indicate that __________
A) there is more than one allotropic form of a compound.
B) more than one ionic form of a compound exists.
C) a molecule's electronic structure is an average or superposition of the resonance structures.
D) more than one isotopic form of an element exists in the molecule.
E) the molecule jumps back and forth between two or more different electronic structures.
A) there is more than one allotropic form of a compound.
B) more than one ionic form of a compound exists.
C) a molecule's electronic structure is an average or superposition of the resonance structures.
D) more than one isotopic form of an element exists in the molecule.
E) the molecule jumps back and forth between two or more different electronic structures.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
28
Trends in electronegativity are primarily a result of __________
A) the number of core electrons in an element.
B) an element's atomic number.
C) the effective nuclear charge (Zeff) that an outer electron experiences.
D) the number of valence electrons of an element.
E) the atomic mass.
A) the number of core electrons in an element.
B) an element's atomic number.
C) the effective nuclear charge (Zeff) that an outer electron experiences.
D) the number of valence electrons of an element.
E) the atomic mass.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
29
Indicate which one of the following does not contain a polar covalent bond.
A) I2
B) CO
C) HF
D) NO
E) CN-
A) I2
B) CO
C) HF
D) NO
E) CN-

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
30
In which bond does the H atom have the highest electron density?
A) H-Cl
B) H -Li
C) H -N
D) H -O
E) H -C
A) H-Cl
B) H -Li
C) H -N
D) H -O
E) H -C

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
31
How many lone-pair electrons are on each of the nitrogen atoms in the Lewis structure for dinitrogen tetroxide (N2O4)?
A) 0
B) 2
C) 4
D) 1
E) 3
A) 0
B) 2
C) 4
D) 1
E) 3

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following bonds is most polar?
A) B - H
B) N - H
C) P - H
D) Al - H
E) C - H
A) B - H
B) N - H
C) P - H
D) Al - H
E) C - H

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following species contains a double bond?
A) NO-
B) F2
C) CH
D) OH-
E) Br2
A) NO-
B) F2
C) CH
D) OH-
E) Br2

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
34
Which group of elements is listed in order of increasing electronegativity?
A) F < Cl < Ge < Sn
B) Rb < Ca < Sc < Cs
C) Zr < V < Nb < Ta
D) In < Ga < Al < C
E) Cl < Br < I < F
A) F < Cl < Ge < Sn
B) Rb < Ca < Sc < Cs
C) Zr < V < Nb < Ta
D) In < Ga < Al < C
E) Cl < Br < I < F

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
35
Indicate the element with the largest electronegativity.
A) K
B) Cs
C) Mg
D) B
E) Li
A) K
B) Cs
C) Mg
D) B
E) Li

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following properties is typically used to predict the type of bond that forms between two elements?
A) electronegativity
B) ionization energy
C) atomic radius
D) electron affinity
E) atomic mass
A) electronegativity
B) ionization energy
C) atomic radius
D) electron affinity
E) atomic mass

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following is most likely a polar covalent bond?
A) Na -Cl
B) H -H
C) C -N
D) K -F
E) Ca -O
A) Na -Cl
B) H -H
C) C -N
D) K -F
E) Ca -O

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
38
Indicate the element with the smallest electronegativity.
A) Cl
B) F
C) Be
D) K
E) C
A) Cl
B) F
C) Be
D) K
E) C

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
39
Identify the molecule or ion that contains a polar covalent bond.
A) O2
B) N2
C) CN-
D) H2
E) I2
A) O2
B) N2
C) CN-
D) H2
E) I2

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
40
How many covalent bonds are there in CN-?
A) 1
B) 2
C) 3
D) 0
E) 4
A) 1
B) 2
C) 3
D) 0
E) 4

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
41
What is the formal charge of the nitrogen atom in hydrogen cyanide (HCN)?
A) -1
B) 0
C) 1
D) 5
E) 2
A) -1
B) 0
C) 1
D) 5
E) 2

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
42
The formal charge on the oxygen atoms in the Lewis structure for sulfur dioxide (SO2) that minimizes the formal charges is __________
A) 0 for both oxygen atoms.
B) +1 for both oxygen atoms.
C) -1 for both oxygen atoms.
D) 0 for one oxygen atom, -1 for the other.
E) +1 for one oxygen atom, -1 for the other.
A) 0 for both oxygen atoms.
B) +1 for both oxygen atoms.
C) -1 for both oxygen atoms.
D) 0 for one oxygen atom, -1 for the other.
E) +1 for one oxygen atom, -1 for the other.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
43
Ammonium nitrate (NH4NO3) is often used as a fertilizer. How many reasonable Lewis structures can be drawn for the ammonium ion 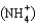 ?
?
A) 0
B) 1
C) 2
D) 3
E) 4
 ?
?A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
44
What is the formal charge on the central nitrogen atom in dinitrogen sulfide (N2S)?
A) 0
B) 1
C) 2
D) -1
E) -2
A) 0
B) 1
C) 2
D) -1
E) -2

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following molecules contains an exception to the octet rule?
A) NO
B) CO
C) HBr
D) I2
E) N2O
A) NO
B) CO
C) HBr
D) I2
E) N2O

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following atoms can have an expanded octet?
A) S
B) C
C) N
D) B
E) Be
A) S
B) C
C) N
D) B
E) Be

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
47
When sulfur-containing fuels are burned, sulfur trioxide (SO3) is formed in the atmosphere and contributes to acid rain (sulfur trioxide plus water produces sulfuric acid). How many resonance structures are there for SO3 when each atom satisfies the octet rule?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
48
How many lone-pair electrons are on the central oxygen atom in the Lewis structure for dinitrogen pentoxide (N2O5)?
A) 0
B) 2
C) 4
D) 1
E) 3
A) 0
B) 2
C) 4
D) 1
E) 3

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
49
What is the total number of electrons assigned to the oxygen atom in the Lewis structure of formaldehyde (H2CO)?
A) 8
B) 6
C) 4
D) 2
E) 10
A) 8
B) 6
C) 4
D) 2
E) 10

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
50
Vinegar is a solution of acetic acid (C2H4O2) and water. Which of the following is the Lewis structure for acetic acid? 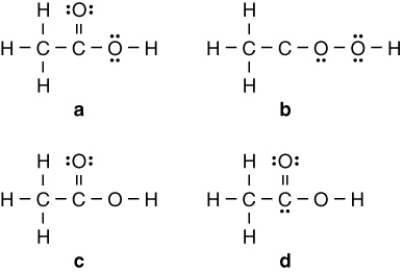
A) a
B) b
C) c
D) d

A) a
B) b
C) c
D) d

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
51
The formal charge of an atom in a Lewis structure is defined as __________
A) the difference between the number of bonding electrons and nonbonding electrons assigned to an atom.
B) the difference between the number of valence electrons in a free atom and the total number of valence electrons in the molecule.
C) the difference between the number of valence electrons in a free atom and the number of electrons assigned to it in a Lewis structure.
D) the difference between the number of valence electrons in a free atom and the number of bonding electrons assigned to it.
E) the difference between the number of valence electrons in a free atom and the number of nonbonding electrons assigned to it.
A) the difference between the number of bonding electrons and nonbonding electrons assigned to an atom.
B) the difference between the number of valence electrons in a free atom and the total number of valence electrons in the molecule.
C) the difference between the number of valence electrons in a free atom and the number of electrons assigned to it in a Lewis structure.
D) the difference between the number of valence electrons in a free atom and the number of bonding electrons assigned to it.
E) the difference between the number of valence electrons in a free atom and the number of nonbonding electrons assigned to it.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
52
How many bonding electrons are assigned to the nitrogen in the Lewis structure of ammonia (NH3)?
A) 3
B) 6
C) 5
D) 8
E) 4
A) 3
B) 6
C) 5
D) 8
E) 4

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
53
Which one of the following molecules or ions contains an exception to the octet rule?
A) O2
B) CN-
C) XeCl
D) ClO-
E) SiH4
A) O2
B) CN-
C) XeCl
D) ClO-
E) SiH4

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
54
What is the formal charge of the carbon atom in the Lewis structure for carbonyl sulfide (OCS)?
A) 0
B) -2
C) 1
D) 4
E) 2
A) 0
B) -2
C) 1
D) 4
E) 2

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following compounds would be the least stable?
A) SF6
B) SeI5
C) CF4Cl
D) PCl2F3
E) CF2Cl2
A) SF6
B) SeI5
C) CF4Cl
D) PCl2F3
E) CF2Cl2

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
56
What types of bonds form between the chlorine and oxygen atoms of the chlorite ion (ClO2-)?
A) single
B) between single and double
C) double
D) triple
E) ionic
A) single
B) between single and double
C) double
D) triple
E) ionic

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
57
Nitrite (NO2-) is an important nutrient in the eutrophic zone of the ocean. Which of the following is the correct set of resonance structures for this ion? 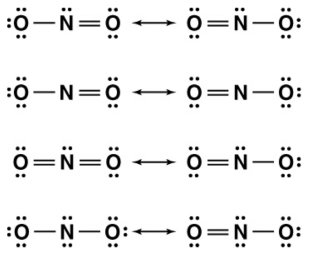
A) a
B) b
C) c
D) d

A) a
B) b
C) c
D) d

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
58
How many total electrons are assigned to the nitrogen atom in the Lewis structure of ammonia (NH3)?
A) 3
B) 6
C) 5
D) 8
E) 4
A) 3
B) 6
C) 5
D) 8
E) 4

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
59
Which structure for dinitrogen sulfide (N -N -S or N -S -N) is the more stable?
A) N -N -S, because the formal charges are all 0
B) N -N -S, because the formal charges are smaller
C) N -S -N, because the formal charges are smaller
D) N -S -N, because the formal charges are all 0
E) N -S -N, because it is symmetrical and the formal charges are the same for both N atoms
A) N -N -S, because the formal charges are all 0
B) N -N -S, because the formal charges are smaller
C) N -S -N, because the formal charges are smaller
D) N -S -N, because the formal charges are all 0
E) N -S -N, because it is symmetrical and the formal charges are the same for both N atoms

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
60
Carbon dioxide is a greenhouse gas. Which of the following is the correct Lewis structure for carbon dioxide?
A)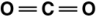
B)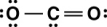
C)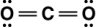
D)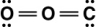
A)
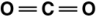
B)
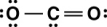
C)
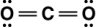
D)
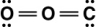

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following is the shortest bond? The number in parentheses is the bond energy.
A) C - C (348 kJ/mol)
B) C - F (453 kJ/mol)
C) C - H (411 kJ/mol)
D) C - Cl (339 kJ/mol)
E) C - O (358 kJ/mol)
A) C - C (348 kJ/mol)
B) C - F (453 kJ/mol)
C) C - H (411 kJ/mol)
D) C - Cl (339 kJ/mol)
E) C - O (358 kJ/mol)

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following bonds will be the shortest?
A) C O
O
B) N O
O
C) S O
O
D) N N
N
A) C
 O
OB) N
 O
OC) S
 O
OD) N
 N
N
Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
63
Based on electronegativities, which one of the following would have ionic bonds?
A) O2
B) MgCl2
C) H2O
D) CF4
E) SiO2
A) O2
B) MgCl2
C) H2O
D) CF4
E) SiO2

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
64
How many valence electrons surround the xenon atom in xenon tetrafluoride (XeF4)?
A) 8
B) 4
C) 10
D) 12
E) 6
A) 8
B) 4
C) 10
D) 12
E) 6

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
65
How many lone-pair electrons are on the central atom of xenon tetrachloride? (XeCl4)?
A) 2
B) 4
C) 8
D) 10
E) 6
A) 2
B) 4
C) 8
D) 10
E) 6

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following is the longest bond? The number in parentheses is the bond energy.
A) C - C (348 kJ/mol)
B) C - F (453 kJ/mol)
C) C - H (411 kJ/mol)
D) C - Cl (339 kJ/mol)
E) C - O (358 kJ/mol)
A) C - C (348 kJ/mol)
B) C - F (453 kJ/mol)
C) C - H (411 kJ/mol)
D) C - Cl (339 kJ/mol)
E) C - O (358 kJ/mol)

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following compounds is likely to have the least reactive hydrogen?
A) H -Br (141 pm)
B) H -I (161 pm)
C) H -Cl (127 pm)
D) H-F (92 pm)
E) H2S (134m)
A) H -Br (141 pm)
B) H -I (161 pm)
C) H -Cl (127 pm)
D) H-F (92 pm)
E) H2S (134m)

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
68
In a chemical reaction, bonds are broken and new bonds are formed. Which one of the following statements regarding bond energies is correct?
A) Energy is released when bonds are broken.
B) Energy is released when bonds are broken and more stable bonds are formed.
C) Energy is released when bonds are broken and less stable bonds are formed.
D) Bond energies and bond lengths generally are not correlated, i.e., they vary independently.
E) Bond energies and bond order are unrelated to each other.
A) Energy is released when bonds are broken.
B) Energy is released when bonds are broken and more stable bonds are formed.
C) Energy is released when bonds are broken and less stable bonds are formed.
D) Bond energies and bond lengths generally are not correlated, i.e., they vary independently.
E) Bond energies and bond order are unrelated to each other.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following compounds would have the largest enthalpy of formation? The bond energies are given in parentheses.
A) H-Br (366 kJ/mol)
B) H -I (299 kJ/mol)
C) H -Cl (431 kJ/mol)
D) H -F (567 kJ/mol)
E) H -H (436 kJ/mol)
A) H-Br (366 kJ/mol)
B) H -I (299 kJ/mol)
C) H -Cl (431 kJ/mol)
D) H -F (567 kJ/mol)
E) H -H (436 kJ/mol)

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
70
Odd electron molecules are called __________
A) anions.
B) cations.
C) radicals.
D) isomers.
E) weird.
A) anions.
B) cations.
C) radicals.
D) isomers.
E) weird.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
71
In which of the following molecules does the central atom have an expanded octet?
A) N2O
B) PCl5
C) BeF2
D) AsF3
E) NO2
A) N2O
B) PCl5
C) BeF2
D) AsF3
E) NO2

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
72
Identify the molecule below that contains a double bond.
A) HCN
B) O3
C) C2H2
D) S2
E) N2
A) HCN
B) O3
C) C2H2
D) S2
E) N2

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
73
Use the formal charge as a criterion to identify the Lewis structure below that is most stable.
A)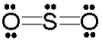
B)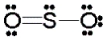
C)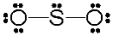
D)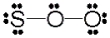
E)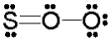
A)
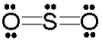
B)
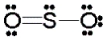
C)
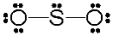
D)

E)
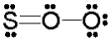

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
74
Bond length and bond strength are __________
A) inversely proportional.
B) directly proportional.
C) identical.
D) unrelated.
E) arbitrary.
A) inversely proportional.
B) directly proportional.
C) identical.
D) unrelated.
E) arbitrary.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
75
How many valence electrons are there in a correctly drawn Lewis structure for formamide, HCONH2?
A) 12
B) 14
C) 16
D) 18
E) 20
A) 12
B) 14
C) 16
D) 18
E) 20

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following oxoacids would you predict not to exist?
A) HFO4
B) HClO4
C) HBrO4
D) HIO4
E) HClO
A) HFO4
B) HClO4
C) HBrO4
D) HIO4
E) HClO

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
77
Acetonitrile (CH3CN) is an important industrial chemical. Among other things, it is used to make plastic moldings, which have multiple uses, from car parts to Lego bricks. Which one of the following statements about acetonitrile is not correct?
A) Acetonitrile has 16 valence electrons.
B) Acetonitrile has one triple bond.
C) Acetonitrile has one pair of nonbonding electrons.
D) All atoms satisfy the octet rule in acetonitrile.
E) One carbon atom and the nitrogen atom have nonzero formal charges.
A) Acetonitrile has 16 valence electrons.
B) Acetonitrile has one triple bond.
C) Acetonitrile has one pair of nonbonding electrons.
D) All atoms satisfy the octet rule in acetonitrile.
E) One carbon atom and the nitrogen atom have nonzero formal charges.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following has the strongest bond? The number in parentheses is the bond length.
A) H - Br (141 pm)
B) H - I (161 pm)
C) H - Cl (127 pm)
D) H - F (92 pm)
E) H - S (134 pm)
A) H - Br (141 pm)
B) H - I (161 pm)
C) H - Cl (127 pm)
D) H - F (92 pm)
E) H - S (134 pm)

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following bonds is the weakest?
A) C -O
B) C O
O
C) O O
O
D) O O
O
A) C -O
B) C
 O
OC) O
 O
OD) O
 O
O
Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
80
An element can form single, double, triple, and quadruple bonds between its atoms. Which will be the shortest?
A) single
B) double
C) triple
D) quadruple
A) single
B) double
C) triple
D) quadruple

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck



