Deck 9: Chemical Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/66
Play
Full screen (f)
Deck 9: Chemical Reactions
1
Which of the following statements concerning redox reactions is incorrect?
A) The oxidizing agent is the substance reduced.
B) The reducing agent is the substance oxidized.
C) The oxidizing agent loses electrons.
D) The substance oxidized loses electrons.
A) The oxidizing agent is the substance reduced.
B) The reducing agent is the substance oxidized.
C) The oxidizing agent loses electrons.
D) The substance oxidized loses electrons.
The oxidizing agent loses electrons.
2
The minimum combined kinetic energy reactant particles must possess in order for their collision to result in a reaction is called the
A) dissociation energy
B) collision energy
C) activation energy
D) orientation energy
A) dissociation energy
B) collision energy
C) activation energy
D) orientation energy
activation energy
3
Combustion reactions are characterized by
A) oxygen always being one of the reactants
B) water always being one of the products
C) carbon dioxide always being one of the products
D) both reactants always being elements
A) oxygen always being one of the reactants
B) water always being one of the products
C) carbon dioxide always being one of the products
D) both reactants always being elements
oxygen always being one of the reactants
4
Which of the following is the correct equilibrium constant expression for the reaction 
A)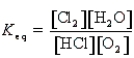
B)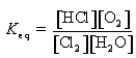
C)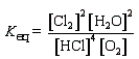
D)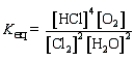

A)
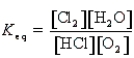
B)
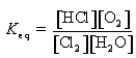
C)
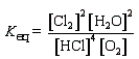
D)
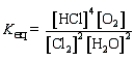

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
5
The proper assignment of oxidation numbers to the elements in the compound LiAsO3 would be
A) +1 for Li, +5 for As and -2 for O
B) +1 for Li, +5 for As and -6 for O
C) +1 for Li, +1 for As and -2 for O
D) +2 for Li, +4 for As and -6 for O
A) +1 for Li, +5 for As and -2 for O
B) +1 for Li, +5 for As and -6 for O
C) +1 for Li, +1 for As and -2 for O
D) +2 for Li, +4 for As and -6 for O

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
6
Most reactions are carried out in liquid solution or in the gaseous phase because in such situations
A) Activation energies are higher.
B) It is easier for reactants to come in contact with each other.
C) Kinetic energies of reactants are lower.
D) Products are less apt to decompose.
A) Activation energies are higher.
B) It is easier for reactants to come in contact with each other.
C) Kinetic energies of reactants are lower.
D) Products are less apt to decompose.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
7
According to Le Chatelier's principle, which of the following changes will shift the position of the equilibrium to the left for the reaction 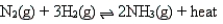
A) double the concentration of N2
B) decrease the concentration of NH3
C) increase the concentration of H2
D) triple the temperature
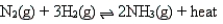
A) double the concentration of N2
B) decrease the concentration of NH3
C) increase the concentration of H2
D) triple the temperature

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
8
The proper assignment of oxidation numbers to the elements in the polyatomic ion SO32- would be
A) +6 for S and -6 for O
B) +6 for S and -2 for O
C) +4 for S and -6 for O
D) +4 for S and -2 for O
A) +6 for S and -6 for O
B) +6 for S and -2 for O
C) +4 for S and -6 for O
D) +4 for S and -2 for O

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
9
Whether a reaction is exothermic or endothermic is determined by
A) the activation energy
B) the physical state of the reactants
C) how reactant and product potential energies compare
D) whether a catalyst is present
A) the activation energy
B) the physical state of the reactants
C) how reactant and product potential energies compare
D) whether a catalyst is present

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
10
Which substance functions as a reducing agent in the following redox reaction? CH4 + 2 O2 CO2 + 2H2O
A) CH4
B) O2
C) CO2
D) H2O
A) CH4
B) O2
C) CO2
D) H2O

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
11
Catalysts are correctly characterized by each of the following statements except one. The exception is:
A) They can be either solids, liquids or gases.
B) They lower the activation energy for a reaction.
C) They do not actively participate in a reaction.
D) They are not consumed in a reaction.
A) They can be either solids, liquids or gases.
B) They lower the activation energy for a reaction.
C) They do not actively participate in a reaction.
D) They are not consumed in a reaction.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
12
What is the change in oxidation number for cobalt in the redox reaction 2Co2O3 + 3C 4Co + 3CO2
A) +4 to -4
B) +2 to 0
C) +3 to +4
D) +3 to 0
A) +4 to -4
B) +2 to 0
C) +3 to +4
D) +3 to 0

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
13
CO2 and H2 are allowed to react until an equilibrium is established as follows: 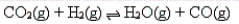 What will be the effect on the equilibrium of removing CO from the equilibrium mixture?
What will be the effect on the equilibrium of removing CO from the equilibrium mixture?
A) The equilibrium will favor the reactants side.
B) H2 concentration will double
C) CO and CO2 concentrations will double
D) H2 concentration will decrease and H2O concentration will increase
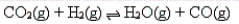 What will be the effect on the equilibrium of removing CO from the equilibrium mixture?
What will be the effect on the equilibrium of removing CO from the equilibrium mixture?A) The equilibrium will favor the reactants side.
B) H2 concentration will double
C) CO and CO2 concentrations will double
D) H2 concentration will decrease and H2O concentration will increase

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following general reaction types is characterized by there being a single reactant?
A) combination
B) decomposition
C) displacement
D) exchange
A) combination
B) decomposition
C) displacement
D) exchange

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
15
For a system at chemical equilibrium:
A) Forward and reverse reaction rates are equal.
B) Forward and reverse reaction rates are zero.
C) Reactant and product concentrations are equal.
D) Reactant and product concentrations are zero.
A) Forward and reverse reaction rates are equal.
B) Forward and reverse reaction rates are zero.
C) Reactant and product concentrations are equal.
D) Reactant and product concentrations are zero.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following changes is most likely to decrease reaction rate for the chemical reaction 2CO + O2 2CO2?
A) adding twice as much O2 to the reaction mixture
B) tripling the temperature of the reaction mixture
C) adding a catalyst to the reaction mixture
D) removing some CO from the reaction mixture
A) adding twice as much O2 to the reaction mixture
B) tripling the temperature of the reaction mixture
C) adding a catalyst to the reaction mixture
D) removing some CO from the reaction mixture

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following reactions is a nonredox decomposition reaction?
A) 2CuO 2Cu + O2
B) 2KClO3 2KCl + 3 O2
C) CaCO3 CaO + CO2
D) SO2 + H2O H2SO3
A) 2CuO 2Cu + O2
B) 2KClO3 2KCl + 3 O2
C) CaCO3 CaO + CO2
D) SO2 + H2O H2SO3

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
18
Which element is oxidized in the following redox reaction? 2H2S + O2 2H2O + S
A) sulfur in H2S
B) hydrogen in H2S
C) oxygen in O2
D) oxygen in H2O
A) sulfur in H2S
B) hydrogen in H2S
C) oxygen in O2
D) oxygen in H2O

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
19
In the redox reaction 2MnBr3 + SnBr2 2MnBr2 + SnBr4
A) SnBr2 is the oxidizing agent
B) MnBr3 is the substance oxidized
C) Sn undergoes an oxidation number change of two units
D) Mn undergoes an oxidation number change of six units
A) SnBr2 is the oxidizing agent
B) MnBr3 is the substance oxidized
C) Sn undergoes an oxidation number change of two units
D) Mn undergoes an oxidation number change of six units

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
20
Le Chatelier's principle states that:
A) Only exothermic reactions can reach chemical equilibrium.
B) Only reactions in which a catalyst is present can reach chemical equilibrium.
C) If a chemical equilibrium is disturbed, the system will try and reestablish equilibrium.
D) If a chemical equilibrium is disturbed, the temperature of the system will always decrease.
A) Only exothermic reactions can reach chemical equilibrium.
B) Only reactions in which a catalyst is present can reach chemical equilibrium.
C) If a chemical equilibrium is disturbed, the system will try and reestablish equilibrium.
D) If a chemical equilibrium is disturbed, the temperature of the system will always decrease.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
21
Use the following to answer the questions below:
In each of the following multiple-choice questions, characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices:
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: (1) The only information needed to write an equilibrium constant expression is a balanced chemical equation with physical state information for the reaction of concern.
(2) The oxidation number of sulfur is the same in the species H2SO4 and SO42-.
(3) Increasing the concentration of a reactant makes collisions between the reactant and other reactants less likely because of "more crowded conditions."
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
In each of the following multiple-choice questions, characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices:
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: (1) The only information needed to write an equilibrium constant expression is a balanced chemical equation with physical state information for the reaction of concern.
(2) The oxidation number of sulfur is the same in the species H2SO4 and SO42-.
(3) Increasing the concentration of a reactant makes collisions between the reactant and other reactants less likely because of "more crowded conditions."
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
22
Chemical equilibrium is reached in a system when
A) complete conversion of reactants to products has occurred
B) reactant and product concentrations are equal
C) reactants are being consumed at the same rate they are being produced
D) more than one correct response
E) no correct response
A) complete conversion of reactants to products has occurred
B) reactant and product concentrations are equal
C) reactants are being consumed at the same rate they are being produced
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
23
CO2 and H2 are allowed to react until equilibrium is established as follows:  Which of the following changes will cause the equilibrium position to shift to the right?
Which of the following changes will cause the equilibrium position to shift to the right?
A) increase in the concentration of H2
B) decrease in the concentration of CO
C) decrease in the concentration of CO2
D) more than one correct response
E) no correct response
 Which of the following changes will cause the equilibrium position to shift to the right?
Which of the following changes will cause the equilibrium position to shift to the right?A) increase in the concentration of H2
B) decrease in the concentration of CO
C) decrease in the concentration of CO2
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
24
For which of the following equilibrium systems will the equilibrium position shift to the left when a stress of increased pressure (from a volume decrease) is applied?
A)
B)
C)
D) more than one correct response
E) no correct response
A)

B)

C)

D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
25
Use the following to answer the questions below:
In each of the following multiple-choice questions, characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices:
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices:
(1) A chemical system reaches equilibrium at the instant when the rate of formation of products becomes zero.
(2) One reactant and two or more products is a general characteristic of decomposition reactions.
(3) Catalysts increase reaction rate by providing an alternate reaction pathway with a higher activation energy.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
In each of the following multiple-choice questions, characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices:
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices:
(1) A chemical system reaches equilibrium at the instant when the rate of formation of products becomes zero.
(2) One reactant and two or more products is a general characteristic of decomposition reactions.
(3) Catalysts increase reaction rate by providing an alternate reaction pathway with a higher activation energy.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following statements about activation energy is correct?
A) It is the same for all reactions.
B) It is low for reactions that take place rapidly.
C) It is the maximum energy a reacting molecule may possess.
D) More than one correct response.
E) No correct response.
A) It is the same for all reactions.
B) It is low for reactions that take place rapidly.
C) It is the maximum energy a reacting molecule may possess.
D) More than one correct response.
E) No correct response.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
27
Use the following to answer the questions below:
In each of the following multiple-choice questions, characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices:
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: (1) Oxidation and reduction are complementary processes that always occur together.
(2) For a system at chemical equilibrium, decreasing the concentration of a reactant will always shift the equilibrium to the right.
(3) The reactants in a combination reaction must be elements.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
In each of the following multiple-choice questions, characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices:
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: (1) Oxidation and reduction are complementary processes that always occur together.
(2) For a system at chemical equilibrium, decreasing the concentration of a reactant will always shift the equilibrium to the right.
(3) The reactants in a combination reaction must be elements.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
28
In the redox reaction 4Fe + 3 O2 + 12HCl 4FeCl3 + 6H2O
A) HCl is the reducing agent.
B) O2 is the substance oxidized.
C) Fe undergoes an oxidation number decrease of three units per atom.
D) More than one correct response.
E) No correct response.
A) HCl is the reducing agent.
B) O2 is the substance oxidized.
C) Fe undergoes an oxidation number decrease of three units per atom.
D) More than one correct response.
E) No correct response.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
29
Use the following to answer the questions below:
In each of the following multiple-choice questions, characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices:
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices:
(1) All chemical systems will reach equilibrium if enough time elapses.
(2) A substance that functions as a reducing agent is, itself, oxidized.
(3) In a slow reaction, the activation energy is far greater than the average energy content of the reacting particles.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
In each of the following multiple-choice questions, characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices:
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices:
(1) All chemical systems will reach equilibrium if enough time elapses.
(2) A substance that functions as a reducing agent is, itself, oxidized.
(3) In a slow reaction, the activation energy is far greater than the average energy content of the reacting particles.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following statements concerning types of reactions is correct?
A) The reactant in a decomposition reaction must be a compound.
B) The reactants in a combination reaction must be elements.
C) The reactants in a displacement reaction could be two compounds.
D) More than one correct response.
E) No correct response.
A) The reactant in a decomposition reaction must be a compound.
B) The reactants in a combination reaction must be elements.
C) The reactants in a displacement reaction could be two compounds.
D) More than one correct response.
E) No correct response.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
31
In which of the following compounds does Cl have an oxidation number of +5?
A) HClO
B) KClO2
C) Ca(ClO3)2
D) more than one correct response
E) no correct response
A) HClO
B) KClO2
C) Ca(ClO3)2
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
32
Use the following to answer the questions below:
In each of the following multiple-choice questions, characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices:
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: (1) The collision between two reactant molecules does not always result in the formation of reaction products.
(2) The substance oxidized in a reaction may be either a reactant or a product.
(3) At chemical equilibrium, the concentrations of reactants and products cease to change.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
In each of the following multiple-choice questions, characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices:
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: (1) The collision between two reactant molecules does not always result in the formation of reaction products.
(2) The substance oxidized in a reaction may be either a reactant or a product.
(3) At chemical equilibrium, the concentrations of reactants and products cease to change.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
33
In a redox reaction, the oxidizing agent is the substance
A) that is oxidized
B) that loses electrons
C) that contains an element that undergoes a decrease in oxidation number
D) more than one correct response
E) no correct response
A) that is oxidized
B) that loses electrons
C) that contains an element that undergoes a decrease in oxidation number
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following reactions is a non-redox reaction?
A) CaCO3 CaO + CO2
B) 2KClO3 2KCl + 3 O2
C) SO3 + H2O H2SO4
D) more than one correct response
E) no correct response
A) CaCO3 CaO + CO2
B) 2KClO3 2KCl + 3 O2
C) SO3 + H2O H2SO4
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
35
According to Le Chatelier's principle, which of the following effects will occur if NH3 is removed from an equilibrium mixture governed by the equation: 
A) Concentration of N2 will increase.
B) Heat will be generated.
C) Concentration of H2 will decrease.
D) More than one correct response.
E) No correct response.

A) Concentration of N2 will increase.
B) Heat will be generated.
C) Concentration of H2 will decrease.
D) More than one correct response.
E) No correct response.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
36
For a collision between molecules to result in reaction, the molecules must possess a certain minimum energy and
A) exchange electrons
B) interact with a catalyst for at least one second
C) have a favorable orientation relative to each other when they collide
D) more than one correct response
E) no correct response
A) exchange electrons
B) interact with a catalyst for at least one second
C) have a favorable orientation relative to each other when they collide
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
37
In writing an equilibrium constant expression, which of the following is incorrect?
A) Concentrations are always expressed in molarities.
B) Product concentrations are always placed in the numerator of the expression.
C) Concentrations of pure solids and pure liquids are always placed in the denominator of the expression.
D) More than one correct response.
E) No correct response.
A) Concentrations are always expressed in molarities.
B) Product concentrations are always placed in the numerator of the expression.
C) Concentrations of pure solids and pure liquids are always placed in the denominator of the expression.
D) More than one correct response.
E) No correct response.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following statements concerning oxidation and reduction is correct?
A) They cannot occur independently of each other.
B) They accompany all chemical changes.
C) They describe, respectively, the gain and loss of electrons.
D) More than one correct response.
E) No correct response.
A) They cannot occur independently of each other.
B) They accompany all chemical changes.
C) They describe, respectively, the gain and loss of electrons.
D) More than one correct response.
E) No correct response.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
39
Increasing the temperature at which a chemical reaction occurs
A) will lower the activation energy
B) causes more reactant collisions to take place in a given time
C) increases disorder in the system, thus decreasing the reaction rate
D) more than one correct response
E) no correct response
A) will lower the activation energy
B) causes more reactant collisions to take place in a given time
C) increases disorder in the system, thus decreasing the reaction rate
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
40
In which of the following sequences of sulfur-containing ions are the ions arranged in order of decreasing oxidation number for S?
A) SO32-, SO42-, S2-
B) SO42-, S2O32-, S2-
C) S2O32-, SO32-, SO42-
D) more than one correct response
E) no correct response
A) SO32-, SO42-, S2-
B) SO42-, S2O32-, S2-
C) S2O32-, SO32-, SO42-
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
41
For the indicated element, select the correct oxidation number from the response list: oxidation number of C in KHCO3+.
A) +2
B) +3
C) +4
D) +6
A) +2
B) +3
C) +4
D) +6

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
42
For the indicated element, select the correct oxidation number from the response list: oxidation number of Mn in Mn3+.
A) +2
B) +3
C) +4
D) +6
A) +2
B) +3
C) +4
D) +6

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
43
For the indicated element, select the correct oxidation number from the response list: oxidation number of Cr in Cr2O72-+.
A) +2
B) +3
C) +4
D) +6
A) +2
B) +3
C) +4
D) +6

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
44
Consider the following redox equation: SO2 + NO2 SO3 + NO, choose an appropriate chemical formula from the list that identifies the reducing agent.
A) SO2
B) SO3
C) NO
D) NO2
A) SO2
B) SO3
C) NO
D) NO2

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
45
Consider the following redox equation: SO2 + NO2 SO3 + NO, choose an appropriate chemical formula from the list that identifies the substance reduced.
A) SO2
B) SO3
C) NO
D) NO2
A) SO2
B) SO3
C) NO
D) NO2

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
46
For the indicated element, select the correct oxidation number from the response list: oxidation number of Fe in Fe2O3+.
A) +2
B) +3
C) +4
D) +6
A) +2
B) +3
C) +4
D) +6

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
47
Assign the following reaction to one of the reaction classifications given in the response list: 4PH3 + Ni(CO)4 4CO + Ni(PH3)4
A) decomposition
B) combination
C) displacement
D) exchange
A) decomposition
B) combination
C) displacement
D) exchange

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
48
Use the following to answer the questions below:
In each of the following multiple-choice questions, characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices:
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: (1) Decomposition reactions and addition reactions are two terms that describe the same type of reaction.
(2) Orientation relative to one another, at the moment of collision, is always a factor in determining whether a collision is effective.
(3) An increase in temperature will always cause an endothermic reaction at equilibrium to shift in the direction that produces more products.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
In each of the following multiple-choice questions, characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices:
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: (1) Decomposition reactions and addition reactions are two terms that describe the same type of reaction.
(2) Orientation relative to one another, at the moment of collision, is always a factor in determining whether a collision is effective.
(3) An increase in temperature will always cause an endothermic reaction at equilibrium to shift in the direction that produces more products.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
49
Assign the following reaction to one of the reaction classifications given in the response list: 2NO2 + H2O2 2HNO3
A) decomposition
B) combination
C) displacement
D) exchange
A) decomposition
B) combination
C) displacement
D) exchange

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
50
Consider the following redox equation: SO2 + NO2 SO3 + NO, choose an appropriate chemical formula from the list that identifies the substance that loses electrons.
A) SO2
B) SO3
C) NO
D) NO2
A) SO2
B) SO3
C) NO
D) NO2

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
51
Use the following to answer the questions below:
In each of the following multiple-choice questions, characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices:
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: (1) The square brackets associated with a general equilibrium constant expression mean that concentrations must be expressed in terms of molarity.
(2) Pressure changes affect all chemical systems at equilibrium in which gaseous reactants or products are present.
(3) In the compound KMnO4, the oxidation number of Mn is +3.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
In each of the following multiple-choice questions, characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices:
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: (1) The square brackets associated with a general equilibrium constant expression mean that concentrations must be expressed in terms of molarity.
(2) Pressure changes affect all chemical systems at equilibrium in which gaseous reactants or products are present.
(3) In the compound KMnO4, the oxidation number of Mn is +3.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
52
For the indicated element, select the correct oxidation number from the response list: oxidation number of S in H2SO4+.
A) +2
B) +3
C) +4
D) +6
A) +2
B) +3
C) +4
D) +6

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
53
Consider the following redox equation: SO2 + NO2 SO3 + NO, choose an appropriate chemical formula from the list that identifies the oxidizing agent.
A) SO2
B) SO3
C) NO
D) NO2
A) SO2
B) SO3
C) NO
D) NO2

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
54
Use the following to answer the questions below:
In each of the following multiple-choice questions, characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices:
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: (1) Of the known atmospheric greenhouse gases, CO2 is both the most abundant and also the most efficient at preventing heat loss from the Earth.
(2) Ozone is a form of oxygen in which there are three atoms per molecule.
(3) A decrease in body temperature slows down the rate of chemical reactions.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
In each of the following multiple-choice questions, characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices:
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: (1) Of the known atmospheric greenhouse gases, CO2 is both the most abundant and also the most efficient at preventing heat loss from the Earth.
(2) Ozone is a form of oxygen in which there are three atoms per molecule.
(3) A decrease in body temperature slows down the rate of chemical reactions.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
55
Consider the following redox equation: SO2 + NO2 SO3 + NO, choose an appropriate chemical formula from the list that identifies the substance oxidized.
A) SO2
B) SO3
C) NO
D) NO2
A) SO2
B) SO3
C) NO
D) NO2

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
56
Use the following to answer the questions below:
In each of the following multiple-choice questions, characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices:
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: (1) Electron loss is always associated with an increase in oxidation number.
(2) An exothermic reaction occurs when the energy required to break bonds in reactants is less than the energy released by bond formation in the products.
(3) The concentrations of pure liquids and pure solids are never included in an equilibrium constant expression because such concentrations never change.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
In each of the following multiple-choice questions, characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices:
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: (1) Electron loss is always associated with an increase in oxidation number.
(2) An exothermic reaction occurs when the energy required to break bonds in reactants is less than the energy released by bond formation in the products.
(3) The concentrations of pure liquids and pure solids are never included in an equilibrium constant expression because such concentrations never change.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
57
Use the following to answer the questions below:
In each of the following multiple-choice questions, characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices:
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: (1) When reactants are in the solid state, reaction rate increases as temperature increases and decreases as state of subdivision increases.
(2) An oxidizing agent causes oxidation by providing electrons for the other reactant to accept.
(3) The numerical value of an equilibrium constant must always be greater than one.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
In each of the following multiple-choice questions, characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices:
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: (1) When reactants are in the solid state, reaction rate increases as temperature increases and decreases as state of subdivision increases.
(2) An oxidizing agent causes oxidation by providing electrons for the other reactant to accept.
(3) The numerical value of an equilibrium constant must always be greater than one.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
58
Assign the following reaction to one of the reaction classifications given in the response list: Mg + 2HCl MgCl2 + H2
A) decomposition
B) combination
C) displacement
D) exchange
A) decomposition
B) combination
C) displacement
D) exchange

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
59
Assign the following reaction to one of the reaction classifications given in the response list: KCN + HCl HCN + KCl
A) decomposition
B) combination
C) displacement
D) exchange
A) decomposition
B) combination
C) displacement
D) exchange

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
60
Assign the following reaction to one of the reaction classifications given in the response list: K2CO3 K2O + CO2
A) decomposition
B) combination
C) displacement
D) exchange
A) decomposition
B) combination
C) displacement
D) exchange

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
61
Consider the following equilibrim:  Determine the effect on the position of equilibrium when a catalyst is added.
Determine the effect on the position of equilibrium when a catalyst is added.
A) shifts to the right
B) shifts to the left
C) does not shift
D) shifts to both the left and the right
 Determine the effect on the position of equilibrium when a catalyst is added.
Determine the effect on the position of equilibrium when a catalyst is added.A) shifts to the right
B) shifts to the left
C) does not shift
D) shifts to both the left and the right

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
62
Consider the following equilibrim:  Determine the effect on the position of equilibrium when the temperature is decreased.
Determine the effect on the position of equilibrium when the temperature is decreased.
A) shifts to the right
B) shifts to the left
C) does not shift
D) shifts to both the left and the right
 Determine the effect on the position of equilibrium when the temperature is decreased.
Determine the effect on the position of equilibrium when the temperature is decreased.A) shifts to the right
B) shifts to the left
C) does not shift
D) shifts to both the left and the right

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
63
For the following general reaction equation, choose the correct form of the equilibrium constant expression from the response list: 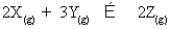
A)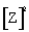
B)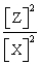
C)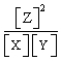
D)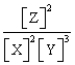
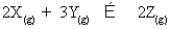
A)
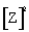
B)
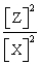
C)
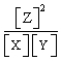
D)
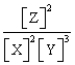

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
64
Consider the following equilibrim:  Determine the effect on the position of equilibrium when the volume of the container is increased (pressure decrease).
Determine the effect on the position of equilibrium when the volume of the container is increased (pressure decrease).
A) shifts to the right
B) shifts to the left
C) does not shift
D) shifts to both the left and the right
 Determine the effect on the position of equilibrium when the volume of the container is increased (pressure decrease).
Determine the effect on the position of equilibrium when the volume of the container is increased (pressure decrease).A) shifts to the right
B) shifts to the left
C) does not shift
D) shifts to both the left and the right

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
65
Consider the following equilibrim:  Determine the effect on the position of equilibrium when some H2 is added.
Determine the effect on the position of equilibrium when some H2 is added.
A) shifts to the right
B) shifts to the left
C) does not shift
D) shifts to both the left and the right
 Determine the effect on the position of equilibrium when some H2 is added.
Determine the effect on the position of equilibrium when some H2 is added.A) shifts to the right
B) shifts to the left
C) does not shift
D) shifts to both the left and the right

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
66
Consider the following equilibrim:  Determine the effect on the position of equilibrium when some CO2 is removed.
Determine the effect on the position of equilibrium when some CO2 is removed.
A) shifts to the right
B) shifts to the left
C) does not shift
D) shifts to both the left and the right
 Determine the effect on the position of equilibrium when some CO2 is removed.
Determine the effect on the position of equilibrium when some CO2 is removed.A) shifts to the right
B) shifts to the left
C) does not shift
D) shifts to both the left and the right

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck



