Deck 5: Chemical Bonding: the Covalent Bond Model
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/70
Play
Full screen (f)
Deck 5: Chemical Bonding: the Covalent Bond Model
1
Which of the following diatomic molecules contains the bond of greatest polarity?
A) N2
B) Cl2
C) ClI
D) BrF
A) N2
B) Cl2
C) ClI
D) BrF
BrF
2
In which of the following molecular compounds are all the bonds single bonds?
A) CS2
B) CS
C) NBr3
D) O2
A) CS2
B) CS
C) NBr3
D) O2
NBr3
3
In which of the following bonds is the bond polarity incorrect?
A)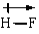
B)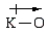
C)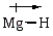
D)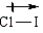
A)
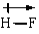
B)
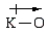
C)
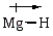
D)
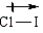
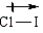
4
For which of the following pairs of elements would the compound formed between them most likely be a molecular compound?
A) Li and Br
B) Ba and O
C) N and S
D) Cs and F
A) Li and Br
B) Ba and O
C) N and S
D) Cs and F

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
5
The correct name for the compound H2Te is
A) hydrogen telluride
B) dihydrogen telluride
C) dihydrogen monotelluride
D) hydrogen monotelluride
A) hydrogen telluride
B) dihydrogen telluride
C) dihydrogen monotelluride
D) hydrogen monotelluride

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
6
The Lewis structure for the polyatomic ion NO2+ contains
A) two double bonds and eight nonbonding electrons
B) one double bond, one single bond, and twelve nonbonding electrons
C) two single bonds and twelve nonbonding electrons
D) two triple bonds and four nonbonding electrons
A) two double bonds and eight nonbonding electrons
B) one double bond, one single bond, and twelve nonbonding electrons
C) two single bonds and twelve nonbonding electrons
D) two triple bonds and four nonbonding electrons

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
7
VSEPR theory predicts that the geometry of the AsH3 molecule is
A) linear
B) trigonal planar
C) trigonal pyramidal
D) tetrahedral
A) linear
B) trigonal planar
C) trigonal pyramidal
D) tetrahedral

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
8
The total number of nonbonding electron pairs present in the Lewis structure of SiH4 is
A) zero
B) one
C) two
D) four
A) zero
B) one
C) two
D) four

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
9
In which of the following molecules is a coordinate covalent bond present?
A) H-C N
B) O=C=O
C) N N
D) N N-O
A) H-C N
B) O=C=O
C) N N
D) N N-O

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following sets of elements is arranged in order of decreasing electronegativity?
A) O, S, As
B) Cl, B, Cl
C) Br, Cl, P
D) Be, C, O
A) O, S, As
B) Cl, B, Cl
C) Br, Cl, P
D) Be, C, O

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following compounds contains both ionic and covalent bonds?
A) SiO2
B) LiI
C) LiCN
D) PCl3
A) SiO2
B) LiI
C) LiCN
D) PCl3

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
12
What is the geometry of the molecular compound formed by the reaction of sulfur with hydrogen?
A) linear
B) angular
C) trigonal planar
D) tetrahedral
A) linear
B) angular
C) trigonal planar
D) tetrahedral

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following statements contrasting covalent bonds and ionic bonds is correct?
A) Covalent bonds usually involve two nonmetals and ionic bonds usually involve two metals.
B) Covalent bonds usually involve two metals and ionic bonds usually involve a metal and a nonmetal.
C) Covalent bonds usually involve a metal and a nonmetal and ionic bonds usually involve two nonmetals.
D) Covalent bonds usually involve two nonmetals and ionic bonds usually involve a metal and a nonmetal.
A) Covalent bonds usually involve two nonmetals and ionic bonds usually involve two metals.
B) Covalent bonds usually involve two metals and ionic bonds usually involve a metal and a nonmetal.
C) Covalent bonds usually involve a metal and a nonmetal and ionic bonds usually involve two nonmetals.
D) Covalent bonds usually involve two nonmetals and ionic bonds usually involve a metal and a nonmetal.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following statements concerning double covalent bonds is correct?
A) They are found only in molecules that contain P or atoms in the third period or higher.
B) They are only found in molecules that contain large atoms that can effectively overlap with each other.
C) They occur only between atoms containing 2 valence electrons.
D) They always involve the sharing of 4 electrons.
A) They are found only in molecules that contain P or atoms in the third period or higher.
B) They are only found in molecules that contain large atoms that can effectively overlap with each other.
C) They occur only between atoms containing 2 valence electrons.
D) They always involve the sharing of 4 electrons.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following molecular compounds would have a Lewis structure that contains 10 electron dots?
A) HCN
B) H2O
C) NH3
D) CO2
A) HCN
B) H2O
C) NH3
D) CO2

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is a molecular compound that contains five atoms per molecule?
A) germanium tetrabromide
B) dinitrogen monoxide
C) dinitrogen pentoxide
D) nitrogen diselenide
A) germanium tetrabromide
B) dinitrogen monoxide
C) dinitrogen pentoxide
D) nitrogen diselenide

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following linear molecules is a nonpolar molecule containing polar bonds?
A) H-C N
B) O=C=O
C) H-Cl
D) N N
A) H-C N
B) O=C=O
C) H-Cl
D) N N

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
18
Given the following electronegativities: Na = 0.9, H = 2.1, Cl = 3.0 which of the following statements is true?
A) H2, Cl2, and HCl are polar covalent molecules.
B) NaCl is a polar covalent compound and HCl is an ionic compound.
C) HCl is a nonpolar covalent compound and Cl2 is a polar covalent molecule.
D) Cl2 is a nonpolar covalent molecule and HCl is a polar covalent molecule.
A) H2, Cl2, and HCl are polar covalent molecules.
B) NaCl is a polar covalent compound and HCl is an ionic compound.
C) HCl is a nonpolar covalent compound and Cl2 is a polar covalent molecule.
D) Cl2 is a nonpolar covalent molecule and HCl is a polar covalent molecule.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
19
Electronegativity is a concept that is useful along with other concepts in
A) predicting the polarity of a bond
B) deciding how many electrons are involved in a bond
C) formulating a statement of the octet rule
D) determining the charge on a polyatomic ion
A) predicting the polarity of a bond
B) deciding how many electrons are involved in a bond
C) formulating a statement of the octet rule
D) determining the charge on a polyatomic ion

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following statements concerning coordinate covalent bonds is correct?
A) One of the atoms involved must be a metal and the other a nonmetal.
B) Both atoms involved in the bond contribute three electrons to the bond.
C) Once formed, they are indistinguishable from any other covalent bond.
D) They occur only in molecules where all bonds are single bonds.
A) One of the atoms involved must be a metal and the other a nonmetal.
B) Both atoms involved in the bond contribute three electrons to the bond.
C) Once formed, they are indistinguishable from any other covalent bond.
D) They occur only in molecules where all bonds are single bonds.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
21
For which of the following pairs of molecular compounds do both members of the pair contain the same number of atoms per molecule?
A) hydrogen peroxide and ammonia
B) hydrazine and methane
C) phosphine and ethane
D) more than one correct response
E) no correct response
A) hydrogen peroxide and ammonia
B) hydrazine and methane
C) phosphine and ethane
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
22
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices provided. (1) Once a coordinate covalent bond is formed, there is no way to distinguish it from any of the other covalent bonds in a molecule.
(2) The least electronegative elements are located to the far right in the periodic table.
(3) The central concept of VSEPR theory is that the electron groups in the valence shell of a central atom in a molecule attract each other.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(2) The least electronegative elements are located to the far right in the periodic table.
(3) The central concept of VSEPR theory is that the electron groups in the valence shell of a central atom in a molecule attract each other.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
23
The number of nonbonding electrons exceeds the number of bonding electrons in the Lewis structure of which of the following molecules?
A) HCN
B) NH3
C) H2O
D) more than one correct response
E) no correct response
A) HCN
B) NH3
C) H2O
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
24
An angular geometry is associated with molecules in which the central atom has
A) two bonds and two nonbonding electron groups
B) two bonds and one nonbonding electron group
C) two bonds and no nonbonding electron groups
D) more than one correct response
E) no correct response
A) two bonds and two nonbonding electron groups
B) two bonds and one nonbonding electron group
C) two bonds and no nonbonding electron groups
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following statements concerning differences between ionic and molecular compounds is correct?
A) Molecular compounds contain two elements, and ionic compounds contain three or more elements.
B) Ionic compounds contain oxygen, and molecular compounds do not.
C) Molecular compounds possess a molecular structural unit, and ionic compounds do not.
D) More than one correct response.
E) No correct response.
A) Molecular compounds contain two elements, and ionic compounds contain three or more elements.
B) Ionic compounds contain oxygen, and molecular compounds do not.
C) Molecular compounds possess a molecular structural unit, and ionic compounds do not.
D) More than one correct response.
E) No correct response.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
26
In which of the following pairs of compounds are both members of the pair molecular compounds?
A) PCl3 and LiBr
B) CCl4 and KOH
C) NaH and CaF2
D) more than one correct response
E) no correct response
A) PCl3 and LiBr
B) CCl4 and KOH
C) NaH and CaF2
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
27
For which of the following pairs of compounds do both members of the pair have names that contain the prefix di-?
A) SCl2 and K2O
B) CO2 and N2O
C) N2O3 and H2S
D) more than one correct response
E) no correct response
A) SCl2 and K2O
B) CO2 and N2O
C) N2O3 and H2S
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
28
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices provided. (1) Nonbonding electron pairs are present on both the H and the F in the molecule HF.
(2) The two atoms involved in a multiple covalent bond must always be atoms of the same element.
(3) A molecule of hydrogen peroxide contains more atoms than does a molecule of ammonia.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(2) The two atoms involved in a multiple covalent bond must always be atoms of the same element.
(3) A molecule of hydrogen peroxide contains more atoms than does a molecule of ammonia.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
29
Sixteen electrons are present in the Lewis structure of which of the following molecules?
A) CO2
B) N2O
C) SO2
D) more than one correct response
E) no correct response
A) CO2
B) N2O
C) SO2
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following tetrahedral molecules is polar?
A) CH4
B) CF4
C) CH3F
D) more than one correct response
E) no correct response
A) CH4
B) CF4
C) CH3F
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
31
Coordinate covalent bonds differ from normal covalent bonds in that:
A) One atom supplies more electrons to the bond than the other atom.
B) The electrons of the bond move about in a coordinated pattern.
C) Atoms involved must always also possess nonbonding electron pairs.
D) More than one correct response.
E) No correct response.
A) One atom supplies more electrons to the bond than the other atom.
B) The electrons of the bond move about in a coordinated pattern.
C) Atoms involved must always also possess nonbonding electron pairs.
D) More than one correct response.
E) No correct response.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
32
If the electronegativity difference between two atoms X and Z is 1.0, the bond between the two atoms would be
A) ionic
B) polar covalent
C) coordinate covalent
D) more than one correct response
E) no correct response
A) ionic
B) polar covalent
C) coordinate covalent
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
33
A period 2 nonmetal, X, forms the molecular compound NX3. Which of the following are also correct formulas for molecular compounds involving X?
A) HX
B) OX2
C) C2X
D) more than one correct response
E) no correct response
A) HX
B) OX2
C) C2X
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
34
In which of the following pairs of triatomic species do both members of the pair have the same geometry?
A) H2O and H2S
B) NO2+ and NO2-
C) HCN and FNO
D) more than one correct response
E) no correct response
A) H2O and H2S
B) NO2+ and NO2-
C) HCN and FNO
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
35
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices provided. (1) The predicted formula of the simplest molecular compound formed between nitrogen and fluorine is NF2.
(2) The majority of compounds contain covalent bonds rather than ionic bonds.
(3) In VSEPR theory, double bonds exert double the influence of single bonds in determining molecular geometry.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(2) The majority of compounds contain covalent bonds rather than ionic bonds.
(3) In VSEPR theory, double bonds exert double the influence of single bonds in determining molecular geometry.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
36
In which of the following triatomic molecules or ions are both of the bonds multiple bonds?
A) HCN
B) CO2
C) NO2-
D) more than one correct response
E) no correct response
A) HCN
B) CO2
C) NO2-
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
37
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices provided. (1) A nitrogen-oxygen bond is more polar than a nitrogen-fluorine bond.
(2) There are no nonbonding electron pairs present on the central atom in a trigonal planar molecule.
(3) The "normal" number of covalent bonds that the element carbon forms is four.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(2) There are no nonbonding electron pairs present on the central atom in a trigonal planar molecule.
(3) The "normal" number of covalent bonds that the element carbon forms is four.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
38
In which of the following pairs of bonds is the first listed bond more polar than the second listed bond?
A) N-N and N-F
B) P-Cl and S-Cl
C) C-N and C-F
D) more than one correct response
E) no correct response
A) N-N and N-F
B) P-Cl and S-Cl
C) C-N and C-F
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
39
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices provided. (1) The number of "electron dots" in the Lewis formula for the compound HNO3 is 24.
(2) Atoms participating in coordinate covalent bonds generally do not form the "normal" number of covalent bonds expected for that element.
(3) Some molecules are nonpolar even though all bonds within the molecules are polar.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(2) Atoms participating in coordinate covalent bonds generally do not form the "normal" number of covalent bonds expected for that element.
(3) Some molecules are nonpolar even though all bonds within the molecules are polar.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following statements concerning molecular polarity is correct?
A) All diatomic molecules are polar.
B) All linear triatomic molecules are nonpolar.
C) Triatomic molecules in which all bonds are polar must be polar.
D) More than one correct response.
E) No correct response.
A) All diatomic molecules are polar.
B) All linear triatomic molecules are nonpolar.
C) Triatomic molecules in which all bonds are polar must be polar.
D) More than one correct response.
E) No correct response.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
41
Compound in which the arrangement of electron groups about the central atom is not tetrahedral
A)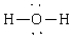
B)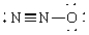
C)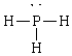
D)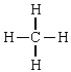
A)

B)
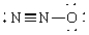
C)
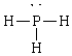
D)


Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
42
Characterize the bond that forms between Li (1.0) and O (3.5).
A) polar covalent bond
B) nonpolar covalent bond
C) coordinate covalent bond
D) ionic bond
A) polar covalent bond
B) nonpolar covalent bond
C) coordinate covalent bond
D) ionic bond

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
43
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices provided. (1) An unpaired electron is present in the Lewis structure for the molecule NO.
(2) The molecule nitric oxide is naturally present in the human body.
(3) The acronym OPR stands for odor replication process.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(2) The molecule nitric oxide is naturally present in the human body.
(3) The acronym OPR stands for odor replication process.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
44
Compound that is nonpolar
A)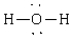
B)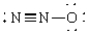
C)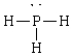
D)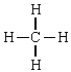
A)

B)
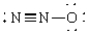
C)
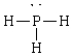
D)


Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
45
Compound that has an angular geometry
A)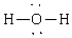
B)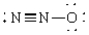
C)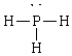
D)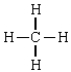
A)

B)
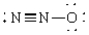
C)
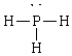
D)


Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
46
Choose the correct number of nonbonding electrons in the Lewis structure for CN- ion.
A) zero
B) one
C) four
D) eight
A) zero
B) one
C) four
D) eight

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
47
Compound with a trigonal pyramidal geometry
A)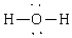
B)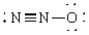
C)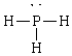
D)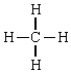
A)

B)
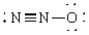
C)
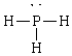
D)


Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
48
Characterize the bond that forms between N (3.0) and Cl (3.0).
A) polar covalent bond
B) nonpolar covalent bond
C) coordinate covalent bond
D) ionic bond
A) polar covalent bond
B) nonpolar covalent bond
C) coordinate covalent bond
D) ionic bond

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
49
Compound that contains both polar and nonpolar bonds
A)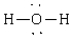
B)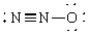
C)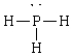
D)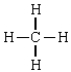
A)

B)
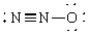
C)
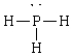
D)


Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
50
Choose the correct number of shared electrons in the Lewis structure for H2O.
A) zero
B) one
C) four
D) eight
A) zero
B) one
C) four
D) eight

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
51
Choose the correct number of double bonds in the Lewis structure for CH4.
A) zero
B) one
C) four
D) eight
A) zero
B) one
C) four
D) eight

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
52
Characterize the bond that forms between N (3.0) and F (4.0).
A) polar covalent bond
B) nonpolar covalent bond
C) coordinate covalent bond
D) ionic bond
A) polar covalent bond
B) nonpolar covalent bond
C) coordinate covalent bond
D) ionic bond

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
53
Characterize the bond that forms between C (2.5) and H (2.1).
A) polar covalent bond
B) nonpolar covalent bond
C) coordinate covalent bond
D) ionic bond
A) polar covalent bond
B) nonpolar covalent bond
C) coordinate covalent bond
D) ionic bond

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
54
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices provided. (1) A pentaatomic tetrahedral molecule may be polar or nonpolar depending on the identities of the atoms present.
(2) A bond between atoms that differ in electronegativity by 2.1 units is classified as a polar covalent bond.
(3) The sum of the number of valence electrons a nonmetallic atom has and the number of covalent bonds it "normally" forms is eight.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(2) A bond between atoms that differ in electronegativity by 2.1 units is classified as a polar covalent bond.
(3) The sum of the number of valence electrons a nonmetallic atom has and the number of covalent bonds it "normally" forms is eight.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
55
Choose the correct number of electrons in the Lewis structure for NH3.
A) zero
B) one
C) four
D) eight
A) zero
B) one
C) four
D) eight

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
56
Choose the correct number of shared electrons contributed by oxygen to the coordinate covalent bond in CO.
A) zero
B) one
C) four
D) eight
A) zero
B) one
C) four
D) eight

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
57
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices provided. (1) In the molecule SO2 both a single bond and a double bond are present.
(2) A linear symmetrical triatomic molecule will always be nonpolar.
(3) The total number of valence electrons available for bonding in the polyatomic ion PO43- is 30.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(2) A linear symmetrical triatomic molecule will always be nonpolar.
(3) The total number of valence electrons available for bonding in the polyatomic ion PO43- is 30.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
58
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices provided. (1) Both tetrahedral and trigonal pyramidal molecules have four VSEPR electron groups about the central atom.
(2) The greater the electronegativity difference between two bonded atoms the greater the polarity of the bond between the two atoms.
(3) A double covalent bond between two atoms is approximately twice as strong as a single covalent bond between the same two atoms.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(2) The greater the electronegativity difference between two bonded atoms the greater the polarity of the bond between the two atoms.
(3) A double covalent bond between two atoms is approximately twice as strong as a single covalent bond between the same two atoms.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
59
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices provided. (1) In the molecule N2 there are more bonding electron pairs than nonbonding electron pairs.
(2) Electronegativity values for sequential period 2 elements differ by 0.5 units.
(3) The octet rule does not apply to covalent compounds in which coordinate covalent bonding is present.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(2) Electronegativity values for sequential period 2 elements differ by 0.5 units.
(3) The octet rule does not apply to covalent compounds in which coordinate covalent bonding is present.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
60
Characterize the bond that forms between Be (1.5) and C (2.5).
A) polar covalent bond
B) nonpolar covalent bond
C) coordinate covalent bond
D) ionic bond
A) polar covalent bond
B) nonpolar covalent bond
C) coordinate covalent bond
D) ionic bond

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
61
A, X, and Z are different elements with different electronegativities. What choice below best describes the polarity of the bonds and molecule in a linear Z-A-X?
A) polar molecule with polar bonds
B) polar molecule with both polar and nonpolar bonds
C) nonpolar molecule with polar bonds
D) nonpolar molecule with nonpolar bonds
A) polar molecule with polar bonds
B) polar molecule with both polar and nonpolar bonds
C) nonpolar molecule with polar bonds
D) nonpolar molecule with nonpolar bonds

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
62
A and X are different elements with different electronegativities. What choice below best describes the polarity of the bonds and molecule in a linear A-A-X?
A) polar molecule with polar bonds
B) polar molecule with both polar and nonpolar bonds
C) nonpolar molecule with polar bonds
D) nonpolar molecule with nonpolar bonds
A) polar molecule with polar bonds
B) polar molecule with both polar and nonpolar bonds
C) nonpolar molecule with polar bonds
D) nonpolar molecule with nonpolar bonds

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
63
Use the following to answer the questions below:
For each of the compounds, select from the response list the correct number of atoms present in a molecule of the compound. Responses on the right may be used more than once or need not be used at all.
a) contains three or fewer atoms
b) contains four atoms
c) contains five atoms
d) contains six or more atoms
Select the correct number of atoms in one molecule of nitrogen dioxide.
A) contains three or fewer atoms
B) contains four atoms
C) contains five atoms
D) contains six or more atoms
For each of the compounds, select from the response list the correct number of atoms present in a molecule of the compound. Responses on the right may be used more than once or need not be used at all.
a) contains three or fewer atoms
b) contains four atoms
c) contains five atoms
d) contains six or more atoms
Select the correct number of atoms in one molecule of nitrogen dioxide.
A) contains three or fewer atoms
B) contains four atoms
C) contains five atoms
D) contains six or more atoms

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
64
A and X are different elements with different electronegativities. What choice below best describes the polarity of the bonds and molecule in a linear A-X-A?
A) polar molecule with polar bonds
B) polar molecule with both polar and nonpolar bonds
C) nonpolar molecule with polar bonds
D) nonpolar molecule with nonpolar bonds
A) polar molecule with polar bonds
B) polar molecule with both polar and nonpolar bonds
C) nonpolar molecule with polar bonds
D) nonpolar molecule with nonpolar bonds

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
65
A, X, and Z are different elements with different electronegativities. What choice below best describes the polarity of the bonds and molecule in a linear A-X-Z?
A) polar molecule with polar bonds
B) polar molecule with both polar and nonpolar bonds
C) nonpolar molecule with polar bonds
D) nonpolar molecule with nonpolar bonds
A) polar molecule with polar bonds
B) polar molecule with both polar and nonpolar bonds
C) nonpolar molecule with polar bonds
D) nonpolar molecule with nonpolar bonds

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
66
Use the following to answer the questions below:
For each of the compounds, select from the response list the correct number of atoms present in a molecule of the compound. Responses on the right may be used more than once or need not be used at all.
a) contains three or fewer atoms
b) contains four atoms
c) contains five atoms
d) contains six or more atoms
Select the correct number of atoms in one molecule of hydrazine.
A) contains three or fewer atoms
B) contains four atoms
C) contains five atoms
D) contains six or more atoms
For each of the compounds, select from the response list the correct number of atoms present in a molecule of the compound. Responses on the right may be used more than once or need not be used at all.
a) contains three or fewer atoms
b) contains four atoms
c) contains five atoms
d) contains six or more atoms
Select the correct number of atoms in one molecule of hydrazine.
A) contains three or fewer atoms
B) contains four atoms
C) contains five atoms
D) contains six or more atoms

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
67
Use the following to answer the questions below:
For each of the compounds, select from the response list the correct number of atoms present in a molecule of the compound. Responses on the right may be used more than once or need not be used at all.
a) contains three or fewer atoms
b) contains four atoms
c) contains five atoms
d) contains six or more atoms
Select the correct number of atoms in one molecule of dichlorine monoxide.
A) contains three or fewer atoms
B) contains four atoms
C) contains five atoms
D) contains six or more atoms
For each of the compounds, select from the response list the correct number of atoms present in a molecule of the compound. Responses on the right may be used more than once or need not be used at all.
a) contains three or fewer atoms
b) contains four atoms
c) contains five atoms
d) contains six or more atoms
Select the correct number of atoms in one molecule of dichlorine monoxide.
A) contains three or fewer atoms
B) contains four atoms
C) contains five atoms
D) contains six or more atoms

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
68
Use the following to answer the questions below:
For each of the compounds, select from the response list the correct number of atoms present in a molecule of the compound. Responses on the right may be used more than once or need not be used at all.
a) contains three or fewer atoms
b) contains four atoms
c) contains five atoms
d) contains six or more atoms
Select the correct number of atoms in one molecule of hydrogen sulfide.
A) contains three or fewer atoms
B) contains four atoms
C) contains five atoms
D) contains six or more atoms
For each of the compounds, select from the response list the correct number of atoms present in a molecule of the compound. Responses on the right may be used more than once or need not be used at all.
a) contains three or fewer atoms
b) contains four atoms
c) contains five atoms
d) contains six or more atoms
Select the correct number of atoms in one molecule of hydrogen sulfide.
A) contains three or fewer atoms
B) contains four atoms
C) contains five atoms
D) contains six or more atoms

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
69
What choice below best describes the polarity of the bonds and molecule in a linear A-A-A?
A) polar molecule with polar bonds
B) polar molecule with both polar and nonpolar bonds
C) nonpolar molecule with polar bonds
D) nonpolar molecule with nonpolar bonds
A) polar molecule with polar bonds
B) polar molecule with both polar and nonpolar bonds
C) nonpolar molecule with polar bonds
D) nonpolar molecule with nonpolar bonds

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
70
Use the following to answer the questions below:
For each of the compounds, select from the response list the correct number of atoms present in a molecule of the compound. Responses on the right may be used more than once or need not be used at all.
a) contains three or fewer atoms
b) contains four atoms
c) contains five atoms
d) contains six or more atoms
Select the correct number of atoms in one molecule of methane.
A) contains three or fewer atoms
B) contains four atoms
C) contains five atoms
D) contains six or more atoms
For each of the compounds, select from the response list the correct number of atoms present in a molecule of the compound. Responses on the right may be used more than once or need not be used at all.
a) contains three or fewer atoms
b) contains four atoms
c) contains five atoms
d) contains six or more atoms
Select the correct number of atoms in one molecule of methane.
A) contains three or fewer atoms
B) contains four atoms
C) contains five atoms
D) contains six or more atoms

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck



