Deck 10: Nuclear Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/85
Play
Full screen (f)
Deck 10: Nuclear Chemistry
1
Which unit describes the amount of radiation absorbed by one gram of a substance?
A)rad
B)rem
C)Bq
D)gray
A)rad
B)rem
C)Bq
D)gray
A
2
What types of cells are most sensitive to radiation damage?
A)the intestinal tract cells
B)reproductive organs cells
C)cancer cells
D)bone marrow cells
E)All of the cell types listed above are very sensitive to radiation damage.
A)the intestinal tract cells
B)reproductive organs cells
C)cancer cells
D)bone marrow cells
E)All of the cell types listed above are very sensitive to radiation damage.
E
3
Phosphorous-32 is used for organ imaging. How many neutrons are present in this radioisotope?
A)15
B)17
C)32
D)47
A)15
B)17
C)32
D)47
B
4
If the half-life of phosphorus-32 is 14.3 days, how much of a 200. mg sample of phosphorus-32 remains after 71.5 days?
A)100. mg
B)50. mg
C)12.5 mg
D)6.25 mg
A)100. mg
B)50. mg
C)12.5 mg
D)6.25 mg

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
5
Which type of radiation has the greatest penetrating power?
A)An alpha particle
B)A gamma ray
C)A beta particle
D)All of the above have the same penetrating power.
A)An alpha particle
B)A gamma ray
C)A beta particle
D)All of the above have the same penetrating power.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
6
Which type of radiation has the largest mass?
A)An alpha particle
B)A gamma ray
C)A positron
D)A beta particle
A)An alpha particle
B)A gamma ray
C)A positron
D)A beta particle

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
7
Which species completes the nuclear equation shown below? 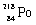 _____ +
_____ + 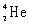
A)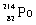
B)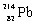
C)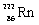
D)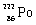
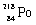 _____ +
_____ + 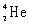
A)
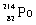
B)
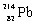
C)
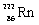
D)
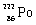

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
8
Which unit of radioactivity is equivalent to 3.7 × 1010 disintegrations/second?
A)1 Ci
B)1 mCi
C)1 Ci
D)1 Bq
A)1 Ci
B)1 mCi
C)1 Ci
D)1 Bq

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
9
What does it mean if a substance is described as being radioactive?
A)The substance emits high energy particles or rays from its unstable nucleus.
B)The substance was exposed to radiation that was given off by a decaying nucleus.
C)The substance readily absorbs energy from the radiofrequency region of the electromagnetic spectrum.
D)The substance is extremely small and special radiomicroscopes are necessary to view it.
A)The substance emits high energy particles or rays from its unstable nucleus.
B)The substance was exposed to radiation that was given off by a decaying nucleus.
C)The substance readily absorbs energy from the radiofrequency region of the electromagnetic spectrum.
D)The substance is extremely small and special radiomicroscopes are necessary to view it.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
10
Which is the symbol for a beta particle?
A)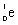
B)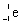
C)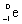
D)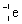
A)
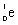
B)
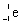
C)
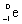
D)
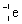

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
11
Which species completes the nuclear equation shown below? 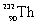
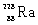 + _____
+ _____
A)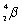
B)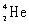
C)2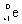
D)2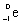
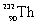
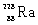 + _____
+ _____A)
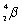
B)
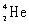
C)2
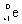
D)2
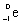

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
12
The half-life (t1/2)of a radioactive isotope depends on which of the following?
A)the amount of the radioisotope present
B)the isotope
C)the temperature
D)the pressure
E)all of the factors listed above
A)the amount of the radioisotope present
B)the isotope
C)the temperature
D)the pressure
E)all of the factors listed above

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
13
Which species completes the nuclear equation shown below? 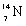 +
+ 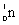 _____ +
_____ + 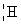
A)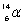
B)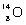
C)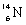
D)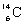
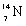 +
+ 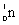 _____ +
_____ + 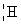
A)
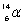
B)
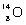
C)
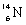
D)
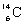

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
14
Which type of radiation has a negligible mass and a charge of +1?
A)alpha particle
B)gamma ray
C)positron
D)beta particle
A)alpha particle
B)gamma ray
C)positron
D)beta particle

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
15
An alpha particle is a high-energy particle that contains
A)one proton and one neutron.
B)two protons and two neutrons.
C)one electron.
D)one proton.
A)one proton and one neutron.
B)two protons and two neutrons.
C)one electron.
D)one proton.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
16
What is the minimum recommended protective equipment when handling a beta particle emitter?
A)lab coat and gloves
B)heavy lab coat and gloves
C)lead shielding
D)No protective equipment is recommended.
A)lab coat and gloves
B)heavy lab coat and gloves
C)lead shielding
D)No protective equipment is recommended.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
17
Which radioisotope is used to measure thyroid activity and treat thyroid disease?
A)Technetium-99m
B)Iodine-131
C)Cobalt-60
D)Carbon-11
A)Technetium-99m
B)Iodine-131
C)Cobalt-60
D)Carbon-11

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
18
To treat a thyroid tumor, a patient must be given a 150-mCi dose of iodine-131, supplied in a solution containing 32 mCi/mL. What volume of solution must be administered per dose?
A)1.0 mL
B)4.7 mL
C)4, 800 mL
D)32 mL
A)1.0 mL
B)4.7 mL
C)4, 800 mL
D)32 mL

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
19
What is the estimated the age of an artifact that has 1/16 of the amount of carbon-14 (relative to carbon-12)compared to living organisms. (C-14 t1/2 = 5, 730 years)
A)358 years
B)5, 730 years
C)17, 190 years
D)22, 920 years
E)28, 650 years
A)358 years
B)5, 730 years
C)17, 190 years
D)22, 920 years
E)28, 650 years

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
20
Which species completes this nuclear equation shown below? 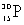 _____ +
_____ + 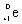
A)
B)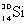
C)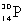
D)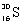
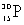 _____ +
_____ + 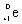
A)

B)
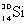
C)
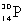
D)
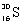

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
21
Which nuclear equation is an example of decay?
A)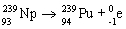
B)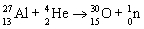
C)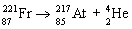
D)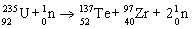
E)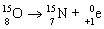
A)
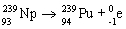
B)
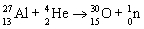
C)
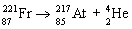
D)
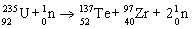
E)

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
22
Which isotope symbol for bismuth is correct?
A)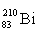
B)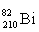
C)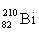
D)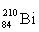
A)
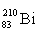
B)
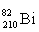
C)
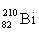
D)
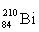

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
23
Beta particles move faster than alpha particles, but they do not penetrate into tissue as far as an alpha particle.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
24
Which quantity represents the greatest amount of radioactivity?
A)1 × 10-3 Ci
B)1 × 104 mCi
C)1 Bq
D)1 × 10-10 Ci
A)1 × 10-3 Ci
B)1 × 104 mCi
C)1 Bq
D)1 × 10-10 Ci

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
25
Mg-24 is a non-radioactive isotope of magnesium and Mg-27 is a radioactive isotope. Which of the following is a true statement concerning these two isotopes?
A)Both isotopes emit high energy particles or rays known as radiation.
B)The radioactive isotope has three more neutrons than the non-radioactive isotope.
C)The radioactive isotope has three more electrons than the non-radioactive isotope.
D)The non-radioactive isotope has three less protons than the radioactive isotope.
A)Both isotopes emit high energy particles or rays known as radiation.
B)The radioactive isotope has three more neutrons than the non-radioactive isotope.
C)The radioactive isotope has three more electrons than the non-radioactive isotope.
D)The non-radioactive isotope has three less protons than the radioactive isotope.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
26
What is the balanced nuclear equation for the emission of the radioisotope strontium-90?
A)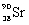
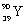 +
+ 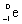
B)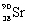
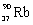 +
+ 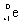
C)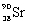
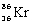 +
+ 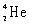
D)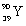 +
+ 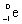
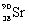
E)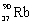 +
+ 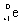
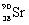
A)
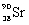
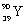 +
+ 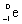
B)
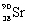
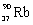 +
+ 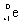
C)
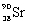
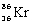 +
+ 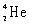
D)
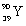 +
+ 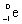
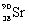
E)
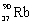 +
+ 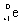

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
27
Which amount of radioactivity is equivalent to 3.7 × 105 mCi?
A)3.7 × 103 Ci
B)3.7 × 102 Ci
C)3.7 × 108 Ci
D)3.7 × 1014 Ci
A)3.7 × 103 Ci
B)3.7 × 102 Ci
C)3.7 × 108 Ci
D)3.7 × 1014 Ci

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
28
Which nuclear equation is an example of positron emission?
A)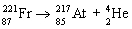
B)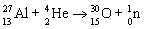
C)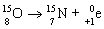
D)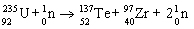
E)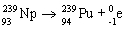
A)
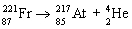
B)
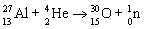
C)
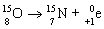
D)
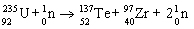
E)
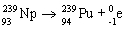

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
29
Which species completes the nuclear equation shown below? 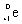 +
+ 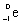 _____
_____
A)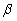
B)
C)
D)2
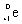 +
+ 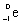 _____
_____A)
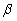
B)
C)
D)2

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
30
The fusion of two helium-3 nuclei produces two protons and what other nucleus? 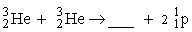
A)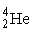
B)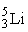
C)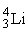
D)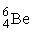
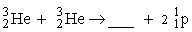
A)
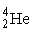
B)
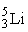
C)
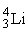
D)
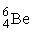

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
31
Which nuclear equation is an example of decay?
A)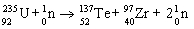
B)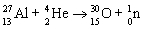
C)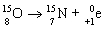
D)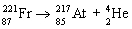
E)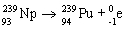
A)
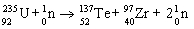
B)
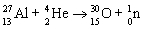
C)
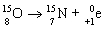
D)
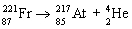
E)
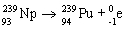

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
32
What is the SI unit in the measurement of radioactivity?
A)Ci
B)mCi
C) Ci
D)Bq
A)Ci
B)mCi
C) Ci
D)Bq

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
33
What are the number of protons, the number of neutrons, and the mass number of the radioisotope 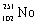 ?
?
A)149, 102, and 251, respectively
B)102, 149, and 251, respectively
C)102, 251, and 353, respectively
D)149, 251, and 353, respectively
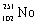 ?
?A)149, 102, and 251, respectively
B)102, 149, and 251, respectively
C)102, 251, and 353, respectively
D)149, 251, and 353, respectively

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
34
Which nuclear equation is an example of nuclear fission?
A)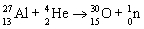
B)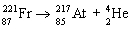
C)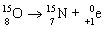
D)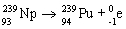
E)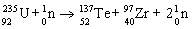
A)
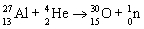
B)
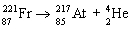
C)
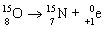
D)
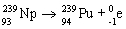
E)
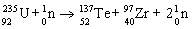

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
35
Which nuclear equation is an example of nuclear fusion?
A)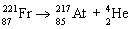
B)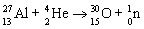
C)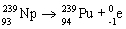
D)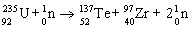
E)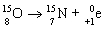
A)
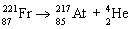
B)
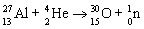
C)
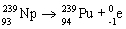
D)
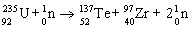
E)
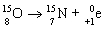

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
36
The initial responders to the Chernobyl nuclear disaster were exposed to 22 Sv of radiation. What is this exposure in units of rem?
A)2.2 mrem
B)2, 200 rem
C)0.022 rem
D)0.22 mrem
A)2.2 mrem
B)2, 200 rem
C)0.022 rem
D)0.22 mrem

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
37
How many neutrons are generated in the nuclear equation shown below? 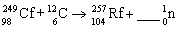
A)0
B)1
C)2
D)3
E)4
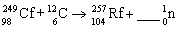
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
38
Which amount of radioactivity is equivalent to 4.1 × 1012 disintegrations/s?
A)1.1 × 103 Ci
B)4.1 × 109 Bq
C)4.1 × 1012 Bq
D)1.1 × 1010 Ci
A)1.1 × 103 Ci
B)4.1 × 109 Bq
C)4.1 × 1012 Bq
D)1.1 × 1010 Ci

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
39
The nuclear equation shown below represents which of the following processes? 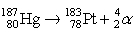
A)alpha decay of platinum
B)gamma emission by mercury
C)alpha emission by mercury
D)gamma decay of platinum
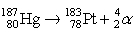
A)alpha decay of platinum
B)gamma emission by mercury
C)alpha emission by mercury
D)gamma decay of platinum

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
40
Which quantity represents the smallest amount of radioactivity?
A)1 × 10-3 Ci
B)1 × 104 mCi
C)1 Bq
D)1 × 1010 Ci
A)1 × 10-3 Ci
B)1 × 104 mCi
C)1 Bq
D)1 × 1010 Ci

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
41
A radiation source used external to the body for therapeutic purposes must have a much shorter half-life than radioisotopes that are ingested for diagnostic purposes.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
42
A nuclear power plant utilizes the tremendous amount of energy produced by fission of the uranium-235 nucleus to heat water to steam, which powers a generator to produce electricity.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
43
A positron is formed when a neutron is converted to a proton and an electron.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
44
Hydrogen-3 is a radioactive isotope of hydrogen. When hydrogen-3 undergoes beta decay, helium-3 is formed.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
45
A microcurie of radioactivity is larger than a Becquerel of radioactivity.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
46
The sum of the mass numbers and the sum of the atomic numbers must be equal on both sides of a nuclear equation.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
47
PET scans are used to detect tumors and coronary artery disease, determine whether cancer has spread to other organs of the body, and monitor whether cancer treatment has been successful.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
48
Nuclear fusion is the splitting apart of a heavy nucleus into lighter nuclei and neutrons.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
49
Radioisotopes that are used for diagnosis and imaging in medicine have short half-lives so they do not linger in the body.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
50
The emission of gamma rays frequently occurs during alpha decay and beta particle emission.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
51
To decrease the incidence of harmful bacteria in foods, certain fruits and vegetables are irradiated with particles that kill any bacteria contained in them.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
52
One rem is equivalent to 100 Sv.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
53
Alpha emission is the decay of a nucleus by emitting an particle, resulting in a new nucleus that has two fewer protons than the original nucleus.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
54
When a positron is emitted from the nucleus of an atom, the nuclear mass remains the same.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
55
The technique of radiocarbon dating is based on the fact that the ratio of radioactive carbon-14 to stable carbon-12 is a constant value in a living organism that is constantly taking in CO2 and other carbon-containing nutrients from its surroundings.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
56
To decrease the incidence of harmful bacteria in foods, certain fruits and vegetables are irradiated with rays that kill any bacteria contained in them.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
57
During gamma emission there is no change in the atomic number or mass number of a radioactive nucleus.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
58
The rad is the amount of radiation that also factors in its energy and potential to damage tissue.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
59
Generally, no detectable biological effects are noticed when the dose of radiation is less than 25 rem.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
60
X-rays, CT scans, and MRIs are techniques that utilize nuclear reactions to provide an image of an organ or extremity that is used for diagnosis of a medical condition.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
61
_____ is the process by which an unstable radioactive nucleus emits radiation, forming a nucleus of new composition.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
62
Nuclear fission reactions generate radioactive waste with long half-lives, often hundreds or even thousands of years.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
63
A gamma ray has twice the mass of an alpha particle.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
64
The balanced nuclear equation for the decay of radon-216 by positron emission is  .
.
 .
.
Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
65
Exposure to 600 rem of radiation is fatal for an entire population.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
66
Red blood cells tagged with technetium-99m are used to find the site of a gastrointestinal bleed.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
67
Nuclear fission and nuclear fusion both release a great deal of energy.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
68
In emission, one neutron of the original nucleus decays to a particle and a(n)_____.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
69
Cobalt-60 is used as an external source of radiation for cancer treatment.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
70
Rb-84 is used to monitor cardiac output and has a half life of 33 days. It would take approximately 165 days for a 1.000 mg sample of Rb-84 to decay to 0.063 mg.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
71
The LD50 of a substance is the lethal dose that kills _____ of a population.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
72
A(n)_____ is a small portable device used for measuring radioactivity.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
73
Only fusion can involve bombarding a nucleus with a neutron.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
74
The balanced nuclear equation for the decay of neon-31 by decay is 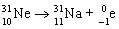 .
.
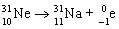 .
. 
Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
75
Isotopes are atoms of the same element having a different number of protons.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
76
An alpha particle is identical to a helium atom.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
77
The light and heat of the sun and other stars is a result of nuclear fusion.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
78
Uranium-235 is used in nuclear weapons. This radioactive isotope contains 92 protons and 235 neutrons.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
79
The balanced nuclear equation for the decay of radon-220 by decay is 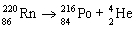 .
.
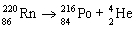 .
. 
Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
80
Two problems that surround nuclear power generation are the possibility of radiation leaks and the disposal of nuclear waste.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck



