Deck 10: Quantity Relationships in Chemical Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/40
Play
Full screen (f)
Deck 10: Quantity Relationships in Chemical Reactions
1
Consider the hypothetical reaction A + 2 B AB2.If 2 moles of A and 5 moles of B are placed in a container an allowed to react,how many moles of AB2 will be formed and how many moles of which reactant will remain unreacted?
A)2 moles of AB2 will form and 3 moles of B will remain unreacted.
B)2 moles of AB2 will form and 1 mole of B will remain unreacted.
C)5 moles of AB2 will form and 3 moles of B will remain unreacted.
D)4 moles of AB2 will form and 1 mole of B will remain unreacted.
E)5/2 (2.5)moles of AB2 will form and nothing will remain unreacted.
A)2 moles of AB2 will form and 3 moles of B will remain unreacted.
B)2 moles of AB2 will form and 1 mole of B will remain unreacted.
C)5 moles of AB2 will form and 3 moles of B will remain unreacted.
D)4 moles of AB2 will form and 1 mole of B will remain unreacted.
E)5/2 (2.5)moles of AB2 will form and nothing will remain unreacted.
2 moles of AB2 will form and 1 mole of B will remain unreacted.
2
Consider the conversion diagram given below. 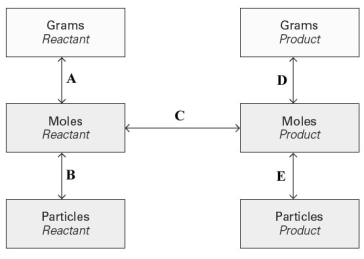 At which point(s)in the diagram is a molar mass needed?
At which point(s)in the diagram is a molar mass needed?
A)A and B
B)B and E
C)A and D
D)D and E
E)C only
 At which point(s)in the diagram is a molar mass needed?
At which point(s)in the diagram is a molar mass needed?A)A and B
B)B and E
C)A and D
D)D and E
E)C only
A and D
3
How many moles of oxygen are consumed in the complete combustion of 1.60 moles of benzene,C6H6?
A)6.00 mol
B)12.0 mol
C)15.0 mol
D)18.0 mol
E)24.0 mol
A)6.00 mol
B)12.0 mol
C)15.0 mol
D)18.0 mol
E)24.0 mol
12.0 mol
4
In reacting aluminum carbonate with hydrochloric acid according to the equation Al2(CO3)3 + 6 HCl 2 AlCl3 + 3 H2O + 3 CO2,57.6 mg of carbon dioxide was formed.What mass of aluminum carbonate reacted to form this amount of carbon dioxide?
A)10.8 mg
B)32.5 mg
C)102 mg
D)306 mg
E)919 mg
A)10.8 mg
B)32.5 mg
C)102 mg
D)306 mg
E)919 mg

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
5
In the reaction P4 + 5 O2 2 P2O5,in order the convert from moles of any substance to moles of another substance as shown by the block diagram.  What is the conversion factor needed?
What is the conversion factor needed?
A)molar mass of a reactant
B)molar mass of a product
C)mole ratio from the equation
D)Avogadro's number
 What is the conversion factor needed?
What is the conversion factor needed?A)molar mass of a reactant
B)molar mass of a product
C)mole ratio from the equation
D)Avogadro's number

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
6
The reaction C6H6 + HNO3 C6H5NO2 + H2O has a 73.5 percent yield under certain conditions.If 12.75 g of C6H6 is used,how much C6H5NO2 will be produced?
A)20.1 g
B)27.4 g
C)8.09 g
D)11.0 g
E)14.8 g
A)20.1 g
B)27.4 g
C)8.09 g
D)11.0 g
E)14.8 g

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following defines the percent yield for a reaction?
A)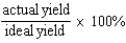
B)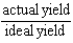
C)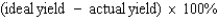
D)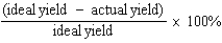
A)
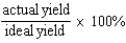
B)
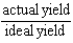
C)
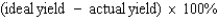
D)
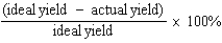

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
8
How many moles of water are produced by burning 5.77 moles of C4H10?
A)1.15 mol
B)5.77 mol
C)23.1 mol
D)28.9 mol
E)37.5 mol
A)1.15 mol
B)5.77 mol
C)23.1 mol
D)28.9 mol
E)37.5 mol

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following best describes a limiting reactant?
A)The reactant that limits the rate (or speed)of a chemical reaction.
B)The reactant that limits the position of equilibrium in a reversible chemical change.
C)The reactant that remains at the end of the reaction.
D)The reactant that can produce the greatest amount of product.
E)The reactant that is completely used up by a reaction.
A)The reactant that limits the rate (or speed)of a chemical reaction.
B)The reactant that limits the position of equilibrium in a reversible chemical change.
C)The reactant that remains at the end of the reaction.
D)The reactant that can produce the greatest amount of product.
E)The reactant that is completely used up by a reaction.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
10
How many moles of bromine will react with 0.0500 mole of C2H2 in the reaction C2H4 + Br2 C2H4Br2?
A)0.100 mol
B)0.186 mol
C)0.0250 mol
D)0.0500 mol
E)2.00 mol
A)0.100 mol
B)0.186 mol
C)0.0250 mol
D)0.0500 mol
E)2.00 mol

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
11
In the reaction CaCN2 + 3 H2O CaCO3 + 2 NH3,how many kilograms of NH3 will be produced when 25.0 kg of CaCN2 react with excess water?
A)10.6 kg
B)12.9 kg
C)2.66 kg
D)5.31 kg
E)7.56 kg
A)10.6 kg
B)12.9 kg
C)2.66 kg
D)5.31 kg
E)7.56 kg

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
12
Barium chloride was used to precipitate silver chloride from a solution of silver nitrate.What mass of barium chloride had to react if 0.635 g of silver chloride formed?
A)0.437 g
B)0.461 g
C)0.874 g
D)0.923 g
E)1.85 g
A)0.437 g
B)0.461 g
C)0.874 g
D)0.923 g
E)1.85 g

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
13
In the reaction 2 AgI + HgI2 Ag2HgI4,2.00 g of AgI and 3.50 g of HgI2 were used.What is the limiting reactant?
A)AgI
B)HgI2
C)Ag2HgI4
D)There is no limiting reactant.
A)AgI
B)HgI2
C)Ag2HgI4
D)There is no limiting reactant.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
14
A chemical reaction resulted in 1.376 g of product,which represented a 78.5 percent yield for the reaction.What is the theoretical yield?
A)0.785 g
B)1.08 g
C)10.8 g
D)1.75 g
E)2.16 g
A)0.785 g
B)1.08 g
C)10.8 g
D)1.75 g
E)2.16 g

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
15
In the complete combustion of C3H8O3,how many moles of carbon dioxide are produced when 23.0 g of C3H8O3 burns?
A)0.250 mol
B)0.750 mol
C)0.0833 mol
D)3.00 mol
E)33.0 mol
A)0.250 mol
B)0.750 mol
C)0.0833 mol
D)3.00 mol
E)33.0 mol

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
16
Silver nitrate and magnesium chloride solutions are mixed.What is the Per expression that allows one to convert moles of silver nitrate to moles of magnesium chloride?
A)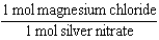
B)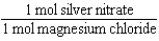
C)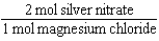
D)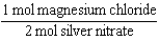
A)
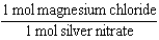
B)
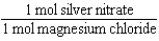
C)
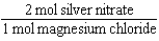
D)
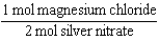

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
17
PCl5 can be produced by the reaction PCl3 + Cl2 PCl5.What mass of PCl3 must be used to produce 127 g of PCl5 if the percent yield is 84.8%?
A)71.1 g
B)83.8 g
C)98.8 g
D)108 g
E)150 g
A)71.1 g
B)83.8 g
C)98.8 g
D)108 g
E)150 g

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
18
How many moles of C6H12O6 are formed when 0.250 mole of CO2 is consumed in the reaction 6 CO2 + 6 H2O C6H12O6 + 6 O2?
A)1.50 mol
B)1.00 mol
C)0.250 mol
D)0.167 mol
E)0.0417 mol
A)1.50 mol
B)1.00 mol
C)0.250 mol
D)0.167 mol
E)0.0417 mol

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
19
The reaction 2 Sb + 3 Cl2 2 SbCl3 has an 88.3% yield.If 4.07 g of SbCl3 is desired,what mass of chlorine should be used?
A)2.15 g
B)0.957 g
C)1.68 g
D)1.90 g
E)3.00 g
A)2.15 g
B)0.957 g
C)1.68 g
D)1.90 g
E)3.00 g

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
20
In the combustion of natural gas according to the equation CH4 + 2 O2 CO2 + 2 H2O,how many grams of water are formed during the combustion of 0.264 mole of CH4?
A)0.528 g
B)2.00 g
C)2.38 g
D)4.75 g
E)9.50 g
A)0.528 g
B)2.00 g
C)2.38 g
D)4.75 g
E)9.50 g

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
21
When one mole of gaseous hydrogen peroxide,H2O2,is made from hydrogen and oxygen gases,the enthalpy change is -136 kJ.Which of the following correctly represents the thermochemical equation?
(i)H2(g)+ O2(g) H2O2(g)+ 136 kJ
(ii)H2(g)+ O2(g)+ 136 kJ H2O2(g)
(iii)H2(g)+ O2(g) H2O2(g)?0?H = -136 kJ
(iv)H2(g)+ O2(g) H2O2(g)?0?H = +136 kJ
A)i only
B)ii only
C)iii only
D)i and iii
E)ii and iv
(i)H2(g)+ O2(g) H2O2(g)+ 136 kJ
(ii)H2(g)+ O2(g)+ 136 kJ H2O2(g)
(iii)H2(g)+ O2(g) H2O2(g)?0?H = -136 kJ
(iv)H2(g)+ O2(g) H2O2(g)?0?H = +136 kJ
A)i only
B)ii only
C)iii only
D)i and iii
E)ii and iv

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
22
Ammonium sulfate can be made by the reaction H2SO4 + 2 NH3 (NH4)2SO4.What quantity of ammonium sulfate will result from the reaction of 5.00 kg of ammonia and 20.0 kg of sulfuric acid?
A)13.5 kg
B)19.4 kg
C)25.0 kg
D)26.9 kg
E)38.8 kg
A)13.5 kg
B)19.4 kg
C)25.0 kg
D)26.9 kg
E)38.8 kg

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
23
Formation of two moles of hydrogen chloride gas from the gases hydrogen and chlorine is a reaction for which H is -185 kJ.Which of the following statements is correct?
(i)The reaction is exothermic.
(ii)Enthalpy of the system decreases.
(iii)Heat is a "product".
A)All are correct
B)All are incorrect
C)i only
D)ii only
E)iii only
(i)The reaction is exothermic.
(ii)Enthalpy of the system decreases.
(iii)Heat is a "product".
A)All are correct
B)All are incorrect
C)i only
D)ii only
E)iii only

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
24
How much energy is required to produce 1.00 kg of aluminum by the reaction 2 Al2O3(s)+ 3 C(s)+ 1.97 * 103 kJ 4 Al(s)+ 3 CO2(g)?
A)5.50 * 10-5 kJ
B)7.52 * 10-2 kJ
C)2.70 *103 kJ
D)7.30 * 104 kJ
E)1.83 * 104 kJ
A)5.50 * 10-5 kJ
B)7.52 * 10-2 kJ
C)2.70 *103 kJ
D)7.30 * 104 kJ
E)1.83 * 104 kJ

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
25
H = +572 kJ for the decomposition of water by the reaction 2 H2O( 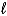 ) 2 H2(g)+ O2(g).How many grams of water can be decomposed by 5.00 *103 kJ of energy?
) 2 H2(g)+ O2(g).How many grams of water can be decomposed by 5.00 *103 kJ of energy?
A)157 g
B)315 g
C)0.0629 g
D)2.57* 107 g
E)5.15 * 107 g
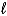 ) 2 H2(g)+ O2(g).How many grams of water can be decomposed by 5.00 *103 kJ of energy?
) 2 H2(g)+ O2(g).How many grams of water can be decomposed by 5.00 *103 kJ of energy?A)157 g
B)315 g
C)0.0629 g
D)2.57* 107 g
E)5.15 * 107 g

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
26
The metabolism of glucose can be represented by the equation C6H12O6(s)+ 6 O2(g) 6 CO2(g)+ 6 H2O(g) H = -2.82 *103 kJ.How many grams of glucose must be metabolized to produce 282 kJ of energy?
A)18.0 g
B)1.80 * 102 g
C)0.100 g
D)10.0 g
E)67.4 g
A)18.0 g
B)1.80 * 102 g
C)0.100 g
D)10.0 g
E)67.4 g

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
27
Cu2HgI4 is prepared according to the equation 2 CuI + HgI2 Cu2HgI4.When 2.00 g of each reactant are used,which one is the limiting reactant?
A)Both reactants are limiting
B)This reaction has no limiting reactant
C)CuI
D)HgI2
E)Cu2HgI4
A)Both reactants are limiting
B)This reaction has no limiting reactant
C)CuI
D)HgI2
E)Cu2HgI4

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
28
A typical diet provides about 2000 food Calories per day,or 2000 kilocalories.Calculate the equivalent number of joules.
A)8 J
B)8 *103 J
C)8 * 106 J
D)0.5 J
E)5 * 105 J
A)8 J
B)8 *103 J
C)8 * 106 J
D)0.5 J
E)5 * 105 J

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
29
A typical candy bar contains 281 food Calories,which is 281 kcal.How many kilojoules are contained in one such candy bar?
A)1.18 * 103 kJ
B)1.18 *104 kJ
C)67.2 kJ
D)672 kJ
E)6.72 * 104 kJ
A)1.18 * 103 kJ
B)1.18 *104 kJ
C)67.2 kJ
D)672 kJ
E)6.72 * 104 kJ

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
30
When 1.00 g of C6H12O6(s)is formed during photosynthesis,15.67 kJ of the sun's energy is converted to chemical energy.What is the thermochemical equation for the photosynthesis of glucose?
A)6 CO2(g)+ 6 H2O(g) C6H12O6(s)+ 6 O2(g)+ 15.67 kJ
B)CO2(g)+ H2O(g) C6H12O6(s)+ O2(g)?0?H = 15.67 kJ
C)6 CO2(g)+ 6 H2O(g)+ 2.82 *103 kJ C6H12O6(s)+ 6 O2(g)
D)C6H12O6(s)+ 6 O2(g) 6 CO2(g)+ 6 H2O(g)+ 2.82*103 kJ
E)6 CO2(g)+ 6 H2O(g) C6H12O6(s)+ 6 O2(g)?0?H = -2.82 * 103 kJ
A)6 CO2(g)+ 6 H2O(g) C6H12O6(s)+ 6 O2(g)+ 15.67 kJ
B)CO2(g)+ H2O(g) C6H12O6(s)+ O2(g)?0?H = 15.67 kJ
C)6 CO2(g)+ 6 H2O(g)+ 2.82 *103 kJ C6H12O6(s)+ 6 O2(g)
D)C6H12O6(s)+ 6 O2(g) 6 CO2(g)+ 6 H2O(g)+ 2.82*103 kJ
E)6 CO2(g)+ 6 H2O(g) C6H12O6(s)+ 6 O2(g)?0?H = -2.82 * 103 kJ

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
31
If the reaction N2 + 3 H2 2 NH3 is carried out using 1.40 g of N2 and 0.400 g of H2,what mass of excess reactant will remain?
A)0.200 g N2
B)1.00 g N2
C)0.100 g H2
D)0.300 g H2
E)Both reactants will be completely consumed
A)0.200 g N2
B)1.00 g N2
C)0.100 g H2
D)0.300 g H2
E)Both reactants will be completely consumed

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
32
The thermochemical equation describing the heat change in the decomposition of limestone,CaCO3,is given by CaCO3(s)+ 176 kJ CaO(s)+ CO2(g).What is the heat change that accompanies the decomposition of 25.0 g of limestone?
A)7.12 kJ
B)25.0 kJ
C)44.0 kJ
D)176 kJ
E)1.42 *10-3 kJ
A)7.12 kJ
B)25.0 kJ
C)44.0 kJ
D)176 kJ
E)1.42 *10-3 kJ

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
33
Consider the following thermochemical equation: CaO(s)+ H2O( 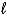 ) Ca(OH)2(s) H = -66.5 kJ Which of the following statements is/are correct?
) Ca(OH)2(s) H = -66.5 kJ Which of the following statements is/are correct?
(i)The reaction is exothermic
(ii)The reaction releases 66.5 kJ per gram of Ca(OH)2(s)formed
(iii)Alternatively,the H term could be added to the product side of the equation
A)i only
B)ii only
C)i and ii
D)i and iii
E)i,ii,and iii
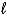 ) Ca(OH)2(s) H = -66.5 kJ Which of the following statements is/are correct?
) Ca(OH)2(s) H = -66.5 kJ Which of the following statements is/are correct?(i)The reaction is exothermic
(ii)The reaction releases 66.5 kJ per gram of Ca(OH)2(s)formed
(iii)Alternatively,the H term could be added to the product side of the equation
A)i only
B)ii only
C)i and ii
D)i and iii
E)i,ii,and iii

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
34
Convert 357 J to kcal.
A)1.49 kcal
B)1.49 * 106 kcal
C)8.53 *104 kcal
D)85.3 kcal
E)0.0853 kcal
A)1.49 kcal
B)1.49 * 106 kcal
C)8.53 *104 kcal
D)85.3 kcal
E)0.0853 kcal

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
35
The decomposition of potassium chlorate is written as shown below.What energy change occurs when 12.3 g of KClO3 decompose? 2 KClO3(s)+ 89.6 kJ 2 KCl(s)+ 3 O2(g)
A)Release of 8.99 kJ
B)Absorption of 8.99 kJ
C)Release of 4.49 kJ
D)Absorption of 4.49 kJ
E)Release of 0.00112 kJ
A)Release of 8.99 kJ
B)Absorption of 8.99 kJ
C)Release of 4.49 kJ
D)Absorption of 4.49 kJ
E)Release of 0.00112 kJ

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
36
Aqueous HCl is added to an aqueous solution of NaOH at 25 °C and the result is shown in the figure below.  Which of the following correctly characterizes this reaction?
Which of the following correctly characterizes this reaction?
A)The reaction is exothermic.
B)The H for the reaction has a + sign.
C)Energy could be considered to be a reactant in the equation for the reaction.
D)The energy (enthalpy)of the products is greater than the reactants.
E)This reaction consumes energy.
 Which of the following correctly characterizes this reaction?
Which of the following correctly characterizes this reaction?A)The reaction is exothermic.
B)The H for the reaction has a + sign.
C)Energy could be considered to be a reactant in the equation for the reaction.
D)The energy (enthalpy)of the products is greater than the reactants.
E)This reaction consumes energy.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
37
A chemist combines hydrochloric acid with a sodium hydroxide solution,transferring 5.75 kJ of heat.Express this in calories.
A)2.41* 10-2 cal
B)2.41* 101 cal
C)2.41 *104 cal
D)1.37 *10-3 cal
E)1.37 * 103 cal
A)2.41* 10-2 cal
B)2.41* 101 cal
C)2.41 *104 cal
D)1.37 *10-3 cal
E)1.37 * 103 cal

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
38
Calculate the mass of Na2O that can be produced by the reaction of 4.00 g of sodium and 2.00 g of oxygen in the reaction 4 Na + O2 2 Na2O.
A)1.94 g
B)2.00 g
C)4.50 g
D)5.39 g
E)6.00 g
A)1.94 g
B)2.00 g
C)4.50 g
D)5.39 g
E)6.00 g

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
39
When C2H5Cl(g)is burned in oxygen,chlorine gas is produced in addition to carbon dioxide and water vapor.5145 kJ of heat are released for every four moles of C2H5Cl(g)burned.Which of the following correctly represents the thermochemical equation?
A)4 C2H5Cl(g)+ 5145 kJ + 13 O2(g) 2 Cl2(g)+ 8 CO2(g)+ 10 H2O(g)
B)4 C2H5Cl(g)+ 13 O2(g)+ 5145 kJ 2 Cl2(g)+ 8 CO2(g)+ 10 H2O(g)
C)4 C2H5Cl(g)+ 13 O2(g) (5145 kJ) 2 Cl2(g)+ 8 CO2(g)+ 10 H2O(g)
D)4 C2H5Cl(g)+ 13 O2(g) 2 Cl2(g)+ 8 CO2(g)+ 10 H2O(g)?0?H = +5145 kJ
E)4 C2H5Cl(g)+ 13 O2(g) 2 Cl2(g)+ 8 CO2(g)+ 10 H2O(g)?0?H = -5145 kJ
A)4 C2H5Cl(g)+ 5145 kJ + 13 O2(g) 2 Cl2(g)+ 8 CO2(g)+ 10 H2O(g)
B)4 C2H5Cl(g)+ 13 O2(g)+ 5145 kJ 2 Cl2(g)+ 8 CO2(g)+ 10 H2O(g)
C)4 C2H5Cl(g)+ 13 O2(g) (5145 kJ) 2 Cl2(g)+ 8 CO2(g)+ 10 H2O(g)
D)4 C2H5Cl(g)+ 13 O2(g) 2 Cl2(g)+ 8 CO2(g)+ 10 H2O(g)?0?H = +5145 kJ
E)4 C2H5Cl(g)+ 13 O2(g) 2 Cl2(g)+ 8 CO2(g)+ 10 H2O(g)?0?H = -5145 kJ

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
40
What is the theoretical yield of phosphorus triiodide when 4.50 g of phosphorus is combined with 45.0 g of iodine? The compound is made by the direct combination of the elements.
A)48.7 g
B)49.5 g
C)59.8 g
D)30.0 g
E)39.9 g
A)48.7 g
B)49.5 g
C)59.8 g
D)30.0 g
E)39.9 g

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck



