Deck 7: Chemical Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/169
Play
Full screen (f)
Deck 7: Chemical Reactions
1
What is the term for a type of reaction in which two or more substances are produced from a single compound?
A)combination
B)decomposition
C)double replacement
D)neutralization
E)single replacement
A)combination
B)decomposition
C)double replacement
D)neutralization
E)single replacement
decomposition
2
What is the term for an ionic compound produced from an acid-base reaction?
A)condensate
B)precipitate
C)salt
D)suspension
E)none of the above
A)condensate
B)precipitate
C)salt
D)suspension
E)none of the above
salt
3
What is the term for a compound that releases hydrogen ions when dissolved in water?
A)acid
B)base
C)aqueous salt
D)aqueous solution
E)none of the above
A)acid
B)base
C)aqueous salt
D)aqueous solution
E)none of the above
acid
4
What is the term for a compound that releases hydroxide ions when dissolved in water?
A)acid
B)base
C)aqueous acid
D)aqueous base
E)none of the above
A)acid
B)base
C)aqueous acid
D)aqueous base
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
5
What is the term for a particle composed of two nonmetal atoms?
A)formula unit
B)diatomic molecule
C)monoatomic ion
D)polyatomic ion
E)none of the above
A)formula unit
B)diatomic molecule
C)monoatomic ion
D)polyatomic ion
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
6
What term refers to an insoluble solid substance that is produced from a chemical reaction in aqueous solution?
A)condensate
B)precipitate
C)salt
D)suspension
E)none of the above
A)condensate
B)precipitate
C)salt
D)suspension
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
7
What is the term for a metal that undergoes reaction with water at room temperature?
A)active metal
B)alkali metal
C)alkaline earth metal
D)rare earth metal
E)none of the above
A)active metal
B)alkali metal
C)alkaline earth metal
D)rare earth metal
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
8
What is the general term for a substance dissolved in water?
A)aqueous salt
B)aqueous substance
C)aqueous solution
D)water solution
E)none of the above
A)aqueous salt
B)aqueous substance
C)aqueous solution
D)water solution
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
9
What is the term for a type of reaction in which an acid and a base react to produce a salt and water?
A)combination
B)decomposition
C)double replacement
D)neutralization
E)single replacement
A)combination
B)decomposition
C)double replacement
D)neutralization
E)single replacement

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
10
What is the term for a substance produced from a chemical change?
A)catalyst
B)compound
C)product
D)reactant
E)none of the above
A)catalyst
B)compound
C)product
D)reactant
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
11
What is the term for the process of undergoing a chemical change?
A)chemical equation
B)chemical formula
C)chemical reaction
D)chemical symbol
E)none of the above
A)chemical equation
B)chemical formula
C)chemical reaction
D)chemical symbol
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
12
What is the term for a substance that speeds up a reaction without being permanently changed?
A)accelerator
B)catalyst
C)inhibitor
D)reactant
E)none of the above
A)accelerator
B)catalyst
C)inhibitor
D)reactant
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
13
What is the term for a substance undergoing a chemical change?
A)catalyst
B)compound
C)product
D)reactant
E)none of the above
A)catalyst
B)compound
C)product
D)reactant
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
14
What is the term for the relative order of metals arranged by their ability to undergo reaction?
A)activity series
B)elemental series
C)reactivity series
D)productivity series
E)none of the above
A)activity series
B)elemental series
C)reactivity series
D)productivity series
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
15
What term refers to a chemical reaction that releases heat energy?
A)endothermic
B)exothermic
C)isothermal
D)all of the above
E)none of the above
A)endothermic
B)exothermic
C)isothermal
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
16
What is the term for a type of reaction in which two substances react to produce a single compound?
A)combination
B)decomposition
C)double replacement
D)neutralization
E)single replacement
A)combination
B)decomposition
C)double replacement
D)neutralization
E)single replacement

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
17
What is the term for a type of reaction in which two cations in different compounds exchange anions?
A)combination
B)decomposition
C)double replacement
D)neutralization
E)single replacement
A)combination
B)decomposition
C)double replacement
D)neutralization
E)single replacement

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
18
What term refers to a chemical reaction that absorbs heat energy?
A)endothermic
B)exothermic
C)isothermal
D)all of the above
E)none of the above
A)endothermic
B)exothermic
C)isothermal
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
19
What is the term for a shorthand representation using formulas and symbols to describe a chemical change?
A)chemical equation
B)chemical formula
C)chemical reaction
D)chemical symbol
E)none of the above
A)chemical equation
B)chemical formula
C)chemical reaction
D)chemical symbol
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
20
What is the term for a digit in front of a chemical formula that helps to balance a chemical equation?
A)coefficient
B)exponent
C)subscript
D)superscript
E)none of the above
A)coefficient
B)exponent
C)subscript
D)superscript
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
21
What is the term for a digit in a chemical formula that represents the number of atoms or ions occurring in a substance?
A)coefficient
B)exponent
C)subscript
D)superscript
E)none of the above
A)coefficient
B)exponent
C)subscript
D)superscript
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following elements occurs naturally as diatomic molecules?
A)carbon
B)phosphorus
C)sulfur
D)all of the above
E)none of the above
A)carbon
B)phosphorus
C)sulfur
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the statements below best describes the following reaction? Na₂CO₃(s) 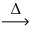 Ni(NO₂)2(s)+ O₂(g)
Ni(NO₂)2(s)+ O₂(g)
A)Nickel(II)nitrate decomposes with heat.
B)Nickel(II)nitrate decomposes to nickel(II)nitrite and oxygen.
C)Nickel(II)nitrate decomposes to nickel(II)nitrite and oxygen gas.
D)Nickel(II)nitrate is heated to give nickel(II)nitrite and oxygen.
E)Solid nickel(II)nitrate is heated to give solid nickel(II)nitrite and oxygen gas.
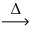 Ni(NO₂)2(s)+ O₂(g)
Ni(NO₂)2(s)+ O₂(g)A)Nickel(II)nitrate decomposes with heat.
B)Nickel(II)nitrate decomposes to nickel(II)nitrite and oxygen.
C)Nickel(II)nitrate decomposes to nickel(II)nitrite and oxygen gas.
D)Nickel(II)nitrate is heated to give nickel(II)nitrite and oxygen.
E)Solid nickel(II)nitrate is heated to give solid nickel(II)nitrite and oxygen gas.

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following is a general guideline for balancing an equation?
A)Write correct formulas for reactants and products.
B)Begin balancing with the most complex formula.
C)Balance polyatomic ions as a single unit.
D)Check each reactant and product to verify the coefficients.
E)all of the above
A)Write correct formulas for reactants and products.
B)Begin balancing with the most complex formula.
C)Balance polyatomic ions as a single unit.
D)Check each reactant and product to verify the coefficients.
E)all of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following formulas represents an element in its natural state?
A)B2
B)C₂
C)N₂
D)P2
E)none of the above
A)B2
B)C₂
C)N₂
D)P2
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following is evidence for a reaction when two solutions mix?
A)Bubbles are released.
B)Insoluble particles form.
C)An indicator changes color.
D)Heat is produced.
E)all of the above
A)Bubbles are released.
B)Insoluble particles form.
C)An indicator changes color.
D)Heat is produced.
E)all of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following is evidence for a chemical reaction?
A)A gas is produced.
B)An insoluble solid is produced in solution.
C)A permanent color change is observed.
D)An energy change is observed.
E)all of the above
A)A gas is produced.
B)An insoluble solid is produced in solution.
C)A permanent color change is observed.
D)An energy change is observed.
E)all of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following is evidence for a chemical reaction?
A)A gas is detected.
B)A precipitate is formed.
C)A flame is observed.
D)all of the above
E)none of the above
A)A gas is detected.
B)A precipitate is formed.
C)A flame is observed.
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following is evidence for a chemical reaction?
A)change in physical state
B)change in color
C)change in mass
D)all of the above
E)none of the above
A)change in physical state
B)change in color
C)change in mass
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the statements below best describes the following reaction? HC₂H₃O₂(aq)+ NaOH(aq)→ NaC₂H₃O₂(aq)+ H₂O(l)
A)An acid plus a base react to produce a salt and water.
B)Acetic acid and sodium hydroxide produce sodium acetate and water.
C)Acetic acid and sodium hydroxide solutions react to produce sodium acetate and water.
D)Acetic acid and sodium hydroxide solutions react to produce sodium acetate solution and water.
E)Aqueous solutions of acetic acid and sodium hydroxide react to produce aqueous sodium acetate and water.
A)An acid plus a base react to produce a salt and water.
B)Acetic acid and sodium hydroxide produce sodium acetate and water.
C)Acetic acid and sodium hydroxide solutions react to produce sodium acetate and water.
D)Acetic acid and sodium hydroxide solutions react to produce sodium acetate solution and water.
E)Aqueous solutions of acetic acid and sodium hydroxide react to produce aqueous sodium acetate and water.

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
31
What is the term for a type of reaction in which a more active metal displaces a less active metal from a solution?
A)combination
B)decomposition
C)double replacement
D)neutralization
E)single replacement
A)combination
B)decomposition
C)double replacement
D)neutralization
E)single replacement

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following formulas represents an element in its natural state?
A)Cl₂
B)Br₂
C)I₂
D)all of the above
E)none of the above
A)Cl₂
B)Br₂
C)I₂
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following elements occurs naturally as diatomic molecules?
A)argon gas
B)helium gas
C)neon gas
D)all of the above
E)none of the above
A)argon gas
B)helium gas
C)neon gas
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the statements below best describes the following reaction? HCl(aq)+ KOH(aq)→ KCl(aq)+ H₂O(l)
A)An acid plus a base react to produce a salt and water.
B)Hydrochloric acid and potassium hydroxide produce potassium chloride and water.
C)Hydrochloric acid and potassium hydroxide solutions react to produce potassium chloride and water.
D)Hydrochloric acid and potassium hydroxide solutions react to produce potassium chloride solution and water.
E)Aqueous solutions of hydrochloric acid and potassium hydroxide react to produce aqueous potassium chloride and water.
A)An acid plus a base react to produce a salt and water.
B)Hydrochloric acid and potassium hydroxide produce potassium chloride and water.
C)Hydrochloric acid and potassium hydroxide solutions react to produce potassium chloride and water.
D)Hydrochloric acid and potassium hydroxide solutions react to produce potassium chloride solution and water.
E)Aqueous solutions of hydrochloric acid and potassium hydroxide react to produce aqueous potassium chloride and water.

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the statements below best describes the following reaction? Na₂CO₃(s) 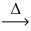 Na₂O(s)+ CO₂(g)
Na₂O(s)+ CO₂(g)
A)Sodium carbonate decomposes with heat.
B)Sodium carbonate decomposes to sodium oxide and carbon dioxide.
C)Sodium carbonate decomposes to sodium oxide and carbon dioxide gas.
D)Sodium carbonate is heated to give sodium oxide and carbon dioxide.
E)Solid sodium carbonate is heated to give solid sodium oxide and carbon dioxide gas.
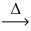 Na₂O(s)+ CO₂(g)
Na₂O(s)+ CO₂(g)A)Sodium carbonate decomposes with heat.
B)Sodium carbonate decomposes to sodium oxide and carbon dioxide.
C)Sodium carbonate decomposes to sodium oxide and carbon dioxide gas.
D)Sodium carbonate is heated to give sodium oxide and carbon dioxide.
E)Solid sodium carbonate is heated to give solid sodium oxide and carbon dioxide gas.

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the statements below best describes the following reaction? HNO₃(aq)+ LiOH(aq)→ LiNO₃(aq)+ H₂O(l)
A)An acid plus a base react to produce a salt and water.
B)Nitric acid and lithium hydroxide produce lithium nitrate and water.
C)Nitric acid and lithium hydroxide solutions react to produce lithium nitrate and water.
D)Nitric acid and lithium hydroxide solutions react to produce lithium nitrate solution and water.
E)Aqueous solutions of nitric acid and lithium hydroxide react to produce aqueous lithium nitrate and water.
A)An acid plus a base react to produce a salt and water.
B)Nitric acid and lithium hydroxide produce lithium nitrate and water.
C)Nitric acid and lithium hydroxide solutions react to produce lithium nitrate and water.
D)Nitric acid and lithium hydroxide solutions react to produce lithium nitrate solution and water.
E)Aqueous solutions of nitric acid and lithium hydroxide react to produce aqueous lithium nitrate and water.

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following elements occurs naturally as diatomic molecules?
A)chlorine gas
B)fluorine gas
C)iodine vapor
D)all of the above
E)none of the above
A)chlorine gas
B)fluorine gas
C)iodine vapor
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following is evidence for a chemical reaction releasing energy?
A)light
B)heat
C)flame
D)explosion
E)all of the above
A)light
B)heat
C)flame
D)explosion
E)all of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following is evidence for a chemical reaction producing a gas?
A)A flaming wooden splint is extinguished.
B)A glowing wooden splint bursts into flame.
C)Two solutions poured together release bubbles.
D)An aged vitamin capsule releases an odor.
E)all of the above
A)A flaming wooden splint is extinguished.
B)A glowing wooden splint bursts into flame.
C)Two solutions poured together release bubbles.
D)An aged vitamin capsule releases an odor.
E)all of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following formulas represents an element in its natural state?
A)H₂
B)N₂
C)O₂
D)all of the above
E)none of the above
A)H₂
B)N₂
C)O₂
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
41
What is the coefficient of oxygen gas after balancing the following equation? __HgO(s) 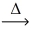 __Hg(s)+ __O₂(g)
__Hg(s)+ __O₂(g)
A)1
B)2
C)3
D)4
E)none of the above
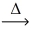 __Hg(s)+ __O₂(g)
__Hg(s)+ __O₂(g)A)1
B)2
C)3
D)4
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
42
What is the coefficient of oxygen gas after balancing the following equation? __H₂O₂(l) 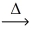 __H₂O(l)+ __O₂(g)
__H₂O(l)+ __O₂(g)
A)1
B)2
C)3
D)4
E)none of the above
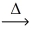 __H₂O(l)+ __O₂(g)
__H₂O(l)+ __O₂(g)A)1
B)2
C)3
D)4
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
43
What is the coefficient of hydrogen gas after balancing the following equation? __Pb(s)+ __HNO₃(aq)→ __Pb(NO₃)4(aq)+ __H₂(g)
A)1
B)2
C)3
D)4
E)none of the above
A)1
B)2
C)3
D)4
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
44
What is the coefficient of phosphorus after balancing the following equation? __P(s)+ __O₂(g)→ __P2O5(s)
A)1
B)2
C)4
D)5
E)none of the above
A)1
B)2
C)4
D)5
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
45
What is the coefficient of carbon dioxide after balancing the following equation? __KHCO₃(s) 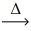 __k₂CO₃(s)+ __H₂O(g)+ __CO₂(g)
__k₂CO₃(s)+ __H₂O(g)+ __CO₂(g)
A)1
B)2
C)3
D)4
E)none of the above
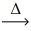 __k₂CO₃(s)+ __H₂O(g)+ __CO₂(g)
__k₂CO₃(s)+ __H₂O(g)+ __CO₂(g)A)1
B)2
C)3
D)4
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
46
What is the coefficient of carbon dioxide after balancing the following equation? __Cr₂(CO₃)3(s) 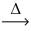 __Cr₂O₃(s)+ __CO₂(g)
__Cr₂O₃(s)+ __CO₂(g)
A)1
B)2
C)3
D)4
E)none of the above
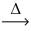 __Cr₂O₃(s)+ __CO₂(g)
__Cr₂O₃(s)+ __CO₂(g)A)1
B)2
C)3
D)4
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
47
What is the coefficient of Cd metal after balancing the following equation? __Al(s)+ __Cd(C₂H₃O₂)2(aq)→ __Al(C₂H₃O₂)3(aq)+ __Cd(s)
A)1
B)2
C)3
D)4
E)none of the above
A)1
B)2
C)3
D)4
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
48
What is the coefficient of oxygen gas after balancing the following equation? __P(s)+ __O₂(g)→ __P2O5(s)
A)1
B)2
C)4
D)5
E)none of the above
A)1
B)2
C)4
D)5
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
49
What is the coefficient of hydrogen gas after balancing the following equation? __Co(s)+ __HCl(aq)→ __CoCl3(aq)+ __H₂(g)
A)1
B)2
C)3
D)4
E)none of the above
A)1
B)2
C)3
D)4
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
50
What is the coefficient of nickel metal after balancing the following equation? __Fe(s)+ __Ni(NO₃)2(aq)→ __Fe(NO₃)3(aq)+ __Ni(s)
A)1
B)2
C)3
D)4
E)none of the above
A)1
B)2
C)3
D)4
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following is not a general guideline for balancing an equation?
A)Write correct formulas for reactants and products.
B)Begin balancing with the most complex formula.
C)Balance polyatomic ions as a single unit.
D)Check each reactant and product to verify the coefficients.
E)Change a subscript in a formula if the equation is not balanced.
A)Write correct formulas for reactants and products.
B)Begin balancing with the most complex formula.
C)Balance polyatomic ions as a single unit.
D)Check each reactant and product to verify the coefficients.
E)Change a subscript in a formula if the equation is not balanced.

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
52
What is the coefficient of chlorine gas after balancing the following equation? __Fe(s)+ __Cl₂(g)→ __FeCl3(s)
A)1
B)2
C)3
D)4
E)none of the above
A)1
B)2
C)3
D)4
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
53
What is the coefficient of nitrogen gas after balancing the following equation? __N₂(g)+ __H₂(g)→ __NH₃(g)
A)1
B)2
C)3
D)4
E)none of the above
A)1
B)2
C)3
D)4
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
54
What is the coefficient of silver metal after balancing the following equation? __Cu(s)+ __AgNO₃(aq)→ __Cu(NO₃)2(aq)+ __Ag(s)
A)1
B)2
C)3
D)4
E)none of the above
A)1
B)2
C)3
D)4
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
55
What is the coefficient of hydrogen gas after balancing the following equation? __N₂(g)+ __H₂(g)→ __NH₃(g)
A)1
B)2
C)3
D)4
E)none of the above
A)1
B)2
C)3
D)4
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
56
What is the coefficient of oxygen gas after balancing the following equation? __P(s)+ __O₂(g)→ __P2O₃(s)
A)1
B)2
C)3
D)5
E)none of the above
A)1
B)2
C)3
D)5
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
57
What is the coefficient of oxygen gas after balancing the following equation? __LiNO₃(s) 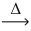 __LiNO₂(s)+ __O₂(g)
__LiNO₂(s)+ __O₂(g)
A)1
B)2
C)3
D)4
E)none of the above
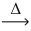 __LiNO₂(s)+ __O₂(g)
__LiNO₂(s)+ __O₂(g)A)1
B)2
C)3
D)4
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
58
What is the coefficient of ammonia gas after balancing the following equation? __N₂(g)+ __H₂(g)→ __NH₃(g)
A)1
B)2
C)3
D)4
E)none of the above
A)1
B)2
C)3
D)4
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
59
What is the coefficient of oxygen gas after balancing the following equation? __AgClO₃(s) 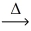 __AgCl(s)+ __O₂(g)
__AgCl(s)+ __O₂(g)
A)1
B)2
C)3
D)4
E)none of the above
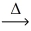 __AgCl(s)+ __O₂(g)
__AgCl(s)+ __O₂(g)A)1
B)2
C)3
D)4
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following is not a general guideline for balancing an equation?
A)Write correct formulas for reactants and products
B)Begin balancing with the most complex formula
C)Balance polyatomic ions as a single unit
D)Balance ionic compounds as a single unit
E)Check each reactant and product to verify the coefficients
A)Write correct formulas for reactants and products
B)Begin balancing with the most complex formula
C)Balance polyatomic ions as a single unit
D)Balance ionic compounds as a single unit
E)Check each reactant and product to verify the coefficients

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
61
What type of chemical reaction is illustrated in the following example? AlCl3(aq)+ AgNO₃(aq)→ Al(NO₃)3(aq)+ AgCl(s)
A)combination reaction
B)decomposition reaction
C)single-replacement reaction
D)double-replacement reaction
E)neutralization reaction
A)combination reaction
B)decomposition reaction
C)single-replacement reaction
D)double-replacement reaction
E)neutralization reaction

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
62
What is the coefficient of water after balancing the following equation? __HC₂H₃O₂(aq)+ __Ca(OH)2(aq)→ __Ca(C₂H₃O₂)2(aq)+ __H₂O(l)
A)1
B)2
C)3
D)6
E)none of the above
A)1
B)2
C)3
D)6
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
63
What is the coefficient of water after balancing the following equation? __H₂SO₄(aq)+ __NaOH(aq)→ __Na₂SO₄(aq)+ __H₂O(l)
A)1
B)2
C)3
D)6
E)none of the above
A)1
B)2
C)3
D)6
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
64
What is the coefficient of water after balancing the following equation? __Li(s)+ __H₂O(l)→ __LiOH(aq)+ __H₂(g)
A)1
B)2
C)3
D)4
E)none of the above
A)1
B)2
C)3
D)4
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
65
What type of chemical reaction is illustrated in the following example? HClO₄(aq)+ KOH(aq)→ KClO₄(aq)+ H₂O(l)
A)combination reaction
B)decomposition reaction
C)single-replacement reaction
D)double-replacement reaction
E)neutralization reaction
A)combination reaction
B)decomposition reaction
C)single-replacement reaction
D)double-replacement reaction
E)neutralization reaction

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
66
What type of chemical reaction is illustrated in the following example? N₂(g)+ H₂(g)→ NH₃(g)
A)combination reaction
B)decomposition reaction
C)single-replacement reaction
D)double-replacement reaction
E)neutralization reaction
A)combination reaction
B)decomposition reaction
C)single-replacement reaction
D)double-replacement reaction
E)neutralization reaction

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
67
What type of chemical reaction is illustrated in the following example? SO₂(g)+ O₂(g)→ SO₃(g)
A)combination reaction
B)decomposition reaction
C)single-replacement reaction
D)double-replacement reaction
E)neutralization reaction
A)combination reaction
B)decomposition reaction
C)single-replacement reaction
D)double-replacement reaction
E)neutralization reaction

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
68
What type of chemical reaction is illustrated in the following example? H₂SO₄(aq)+ NaOH(aq)→ Na₂SO₄(aq)+ H₂O(l)
A)combination reaction
B)decomposition reaction
C)single-replacement reaction
D)double-replacement reaction
E)neutralization reaction
A)combination reaction
B)decomposition reaction
C)single-replacement reaction
D)double-replacement reaction
E)neutralization reaction

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
69
What is the coefficient of sodium metal after balancing the following equation? __Na(s)+ __H₂O(l)→ __NaOH(aq)+ __H₂(g)
A)1
B)2
C)3
D)4
E)none of the above
A)1
B)2
C)3
D)4
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
70
What type of chemical reaction is illustrated in the following example? KClO₃(s) 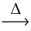 KCl(s)+ O₂(g)
KCl(s)+ O₂(g)
A)combination reaction
B)decomposition reaction
C)single-replacement reaction
D)double-replacement reaction
E)neutralization reaction
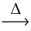 KCl(s)+ O₂(g)
KCl(s)+ O₂(g)A)combination reaction
B)decomposition reaction
C)single-replacement reaction
D)double-replacement reaction
E)neutralization reaction

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
71
What is the coefficient of NaCl after balancing the following equation? __CrCl3(aq)+ __Na₂CO₃(aq)→ __Cr₂(CO₃)3(s)+ __NaCl(aq)
A)1
B)2
C)3
D)6
E)none of the above
A)1
B)2
C)3
D)6
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
72
What is the coefficient of hydrogen gas after balancing the following equation? __Mn(s)+ __H₂SO₄(aq)→ __MnSO₄(aq)+ __H₂(g)
A)1
B)2
C)3
D)4
E)none of the above
A)1
B)2
C)3
D)4
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
73
What is the coefficient of hydrogen gas after balancing the following equation? __Ca(s)+ __H₂O(l)→ __Ca(OH)2(aq)+ __H₂(g)
A)1
B)2
C)3
D)4
E)none of the above
A)1
B)2
C)3
D)4
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
74
What type of chemical reaction is illustrated in the following example? Zn(s)+ HCl(aq)→ ZnCl₂(aq)+ H₂(g)
A)combination reaction
B)decomposition reaction
C)single-replacement reaction
D)double-replacement reaction
E)neutralization reaction
A)combination reaction
B)decomposition reaction
C)single-replacement reaction
D)double-replacement reaction
E)neutralization reaction

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
75
What is the coefficient of AgCl after balancing the following equation? __AlCl3(aq)+ __AgNO₃(aq)→ __Al(NO₃)3(aq)+ __AgCl(s)
A)1
B)2
C)3
D)6
E)none of the above
A)1
B)2
C)3
D)6
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
76
What type of chemical reaction is illustrated in the following example? Sr(s)+ H₂O(l)→ Sr(OH)2(aq)+ H₂(g)
A)combination reaction
B)decomposition reaction
C)single-replacement reaction
D)double-replacement reaction
E)neutralization reaction
A)combination reaction
B)decomposition reaction
C)single-replacement reaction
D)double-replacement reaction
E)neutralization reaction

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
77
What type of chemical reaction is illustrated in the following example? KHCO₃(s) 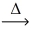 k₂CO₃(s)+ H₂O(g)+ CO₂(g)
k₂CO₃(s)+ H₂O(g)+ CO₂(g)
A)combination reaction
B)decomposition reaction
C)single-replacement reaction
D)double-replacement reaction
E)neutralization reaction
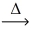 k₂CO₃(s)+ H₂O(g)+ CO₂(g)
k₂CO₃(s)+ H₂O(g)+ CO₂(g)A)combination reaction
B)decomposition reaction
C)single-replacement reaction
D)double-replacement reaction
E)neutralization reaction

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
78
What type of chemical reaction is illustrated in the following example? FeBr3(aq)+ AgNO₃(aq)→ Fe(NO₃)3(aq)+ AgBr(s)
A)combination reaction
B)decomposition reaction
C)single-replacement reaction
D)double-replacement reaction
E)neutralization reaction
A)combination reaction
B)decomposition reaction
C)single-replacement reaction
D)double-replacement reaction
E)neutralization reaction

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
79
What is the coefficient of water after balancing the following equation? __H₃PO₄(aq)+ __Ba(OH)2(aq)→ __Ba3(PO₄)2(s)+ __H₂O(l)
A)1
B)2
C)3
D)6
E)none of the above
A)1
B)2
C)3
D)6
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck
80
What is the coefficient of KNO₃ after balancing the following equation? __Au(NO₃)3(aq)+ __k₂CrO₄(aq)→ __Au2(CrO₄)3(s)+ __KNO₃(aq)
A)1
B)2
C)3
D)6
E)none of the above
A)1
B)2
C)3
D)6
E)none of the above

Unlock Deck
Unlock for access to all 169 flashcards in this deck.
Unlock Deck
k this deck



