Deck 19: Organic Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/191
Play
Full screen (f)
Deck 19: Organic Chemistry
1
What is the term for a hydrocarbon compound typically containing a benzene ring?
A)aromatic hydrocarbon
B)hydrocarbon derivative
C)saturated hydrocarbon
D)unsaturated hydrocarbon
E)none of the above
A)aromatic hydrocarbon
B)hydrocarbon derivative
C)saturated hydrocarbon
D)unsaturated hydrocarbon
E)none of the above
aromatic hydrocarbon
2
What is the term for a family of unsaturated hydrocarbon compounds having a double bond?
A)alkanes
B)alkenes
C)alkynes
D)arenes
E)none of the above
A)alkanes
B)alkenes
C)alkynes
D)arenes
E)none of the above
alkenes
3
What is the term for a chemical reaction in which an unsaturated hydrocarbon adds hydrogen or a halogen?
A)addition reaction
B)combustion reaction
C)halogenation reaction
D)hydrogenation reaction
E)none of the above
A)addition reaction
B)combustion reaction
C)halogenation reaction
D)hydrogenation reaction
E)none of the above
addition reaction
4
What is the term for a compound that contains carbon,hydrogen,and another element such as oxygen,nitrogen,or a halogen?
A)aromatic hydrocarbon
B)hydrocarbon derivative
C)saturated hydrocarbon
D)unsaturated hydrocarbon
E)none of the above
A)aromatic hydrocarbon
B)hydrocarbon derivative
C)saturated hydrocarbon
D)unsaturated hydrocarbon
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
5
What is the term for an atom or group of atoms that characterizes a class of compounds?
A)alkyl group
B)carbonyl group
C)carboxyl group
D)functional group
E)none of the above
A)alkyl group
B)carbonyl group
C)carboxyl group
D)functional group
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
6
What is the term for a small molecule that bonds to itself repeatedly to form a long,continuous chain?
A)monomer
B)polymer
C)saturated hydrocarbon
D)unsaturated hydrocarbon
E)none of the above
A)monomer
B)polymer
C)saturated hydrocarbon
D)unsaturated hydrocarbon
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
7
What is the term for the -OH group?
A)carbonyl group
B)carboxyl group
C)hydroxyl group
D)phenyl group
E)none of the above
A)carbonyl group
B)carboxyl group
C)hydroxyl group
D)phenyl group
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
8
What is the term for the part that remains after a hydrogen atom is removed from an arene?
A)alkyl group
B)aryl group
C)benzyl group
D)ethyl group
E)none of the above
A)alkyl group
B)aryl group
C)benzyl group
D)ethyl group
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
9
What is the term for a family of unsaturated hydrocarbon compounds having a triple bond?
A)alkanes
B)alkenes
C)alkynes
D)arenes
E)none of the above
A)alkanes
B)alkenes
C)alkynes
D)arenes
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
10
What is the term for a family of compounds in which all the members have the same structural feature and similar chemical properties?
A)class of compounds
B)group of compounds
C)functional group
D)isomers
E)none of the above
A)class of compounds
B)group of compounds
C)functional group
D)isomers
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
11
What is the term for the part that remains after a hydrogen atom is removed from a benzene molecule?
A)alkyl group
B)benzyl group
C)ethyl group
D)phenyl group
E)none of the above
A)alkyl group
B)benzyl group
C)ethyl group
D)phenyl group
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
12
What is the term for the -COOH group?
A)carbonyl group
B)carboxyl group
C)hydroxyl group
D)phenyl group
E)none of the above
A)carbonyl group
B)carboxyl group
C)hydroxyl group
D)phenyl group
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
13
What is the current definition of organic chemistry?
A)the study of carbon-containing compounds
B)the study of biologically active compounds
C)the study of plant and animal compounds
D)the study of "vital force" compounds
E)none of the above
A)the study of carbon-containing compounds
B)the study of biologically active compounds
C)the study of plant and animal compounds
D)the study of "vital force" compounds
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
14
What is the term for the C=O group?
A)carbonyl group
B)carboxyl group
C)hydroxyl group
D)phenyl group
E)none of the above
A)carbonyl group
B)carboxyl group
C)hydroxyl group
D)phenyl group
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
15
What is the term for a compound that contains only hydrogen and carbon?
A)aromatic hydrocarbon
B)hydrocarbon
C)saturated hydrocarbon
D)unsaturated hydrocarbon
E)none of the above
A)aromatic hydrocarbon
B)hydrocarbon
C)saturated hydrocarbon
D)unsaturated hydrocarbon
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
16
What is the term for compounds with the same molecular formula,but with different structural formulas?
A)derivatives
B)isostructures
C)isotopes
D)isomers
E)none of the above
A)derivatives
B)isostructures
C)isotopes
D)isomers
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
17
What is the term for the part that remains after a hydrogen atom is removed from an alkane?
A)alkyl group
B)aryl group
C)carbonyl group
D)carboxyl group
E)none of the above
A)alkyl group
B)aryl group
C)carbonyl group
D)carboxyl group
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
18
What is the term for a family of aromatic hydrocarbon compounds?
A)alkanes
B)alkenes
C)alkynes
D)arenes
E)none of the above
A)alkanes
B)alkenes
C)alkynes
D)arenes
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
19
What is the term for a chemical reaction in which a hydrocarbon reacts rapidly with oxygen?
A)addition reaction
B)combustion reaction
C)halogenation reaction
D)hydrogenation reaction
E)none of the above
A)addition reaction
B)combustion reaction
C)halogenation reaction
D)hydrogenation reaction
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
20
What is the term for a family of saturated hydrocarbon compounds?
A)alkanes
B)alkenes
C)alkynes
D)arenes
E)none of the above
A)alkanes
B)alkenes
C)alkynes
D)arenes
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
21
What is the general term for a hydrocarbon that contains a carbon-carbon double bond or triple bond?
A)aromatic hydrocarbon
B)hydrocarbon derivative
C)saturated hydrocarbon
D)unsaturated hydrocarbon
E)none of the above
A)aromatic hydrocarbon
B)hydrocarbon derivative
C)saturated hydrocarbon
D)unsaturated hydrocarbon
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
22
How many isomers share the molecular formula C4H10?
A)2
B)3
C)4
D)5
E)none of the above
A)2
B)3
C)4
D)5
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following hydrocarbons is saturated?
A)alkanes
B)alkenes
C)alkynes
D)all of the above
E)none of the above
A)alkanes
B)alkenes
C)alkynes
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
24
What is the general molecular formula for the alkane class of compounds?
A)CnH₂n
B)CnH₂n+2
C)CnH₂n-2
D)all of the above
E)none of the above
A)CnH₂n
B)CnH₂n+2
C)CnH₂n-2
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following hydrocarbons has a benzene ring?
A)alkanes
B)alkenes
C)arenes
D)all of the above
E)none of the above
A)alkanes
B)alkenes
C)arenes
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
26
What is the IUPAC name for: CH₃-CH₂-CH₂-CH₂-CH₂-CH₃?
A)butane
B)heptane
C)hexane
D)pentane
E)none of the above
A)butane
B)heptane
C)hexane
D)pentane
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following molecular formulas corresponds to an alkane?
A)C₆H10
B)C₆H₁₂
C)C₆H14
D)all of the above
E)none of the above
A)C₆H10
B)C₆H₁₂
C)C₆H14
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following is the condensed structural formula for pentane?
A)CH₃-CH₂-CH₃
B)CH₃-CH₂-CH₂-CH₃
C)CH₃-CH₂-CH₂-CH₂-CH₃
D)CH₃-CH₂-CH₂-CH₂-CH₂-CH₃
E)none of the above
A)CH₃-CH₂-CH₃
B)CH₃-CH₂-CH₂-CH₃
C)CH₃-CH₂-CH₂-CH₂-CH₃
D)CH₃-CH₂-CH₂-CH₂-CH₂-CH₃
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
29
What is the approximate percentage of chemical compounds that are organic?
A)10%
B)25%
C)50%
D)75%
E)90%
A)10%
B)25%
C)50%
D)75%
E)90%

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
30
What is the IUPAC name for: CH₃-CH₂-CH₂-CH₂-CH₂-CH₂-CH₂-CH₃?
A)heptane
B)hexane
C)octane
D)nonane
E)none of the above
A)heptane
B)hexane
C)octane
D)nonane
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following is a fossil fuel?
A)coal
B)natural gas
C)petroleum
D)all of the above
E)none of the above
A)coal
B)natural gas
C)petroleum
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following molecular formulas corresponds to an alkane?
A)C5H₈
B)C5H10
C)C5H₁₂
D)all of the above
E)none of the above
A)C5H₈
B)C5H10
C)C5H₁₂
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following is the condensed structural formula for heptane?
A)CH₃-CH₂-CH₂-CH₂-CH₂-CH₃
B)CH₃-CH₂-CH₂-CH₂-CH₂-CH₂-CH₃
C)CH₃-CH₂-CH₂-CH₂-CH₂-CH₂-CH₂-CH₃
D)CH₃-CH₂-CH₂-CH₂-CH₂-CH₂-CH₂-CH₂-CH₃
E)none of the above
A)CH₃-CH₂-CH₂-CH₂-CH₂-CH₃
B)CH₃-CH₂-CH₂-CH₂-CH₂-CH₂-CH₃
C)CH₃-CH₂-CH₂-CH₂-CH₂-CH₂-CH₂-CH₃
D)CH₃-CH₂-CH₂-CH₂-CH₂-CH₂-CH₂-CH₂-CH₃
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
34
What is the approximate number of identified organic compounds?
A)50,000
B)500,000
C)5,000,000
D)50,000,000
E)500,000,000
A)50,000
B)500,000
C)5,000,000
D)50,000,000
E)500,000,000

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following hydrocarbons are unsaturated?
A)alkanes and alkenes
B)alkanes and alkynes
C)alkenes and alkynes
D)all of the above
E)none of the above
A)alkanes and alkenes
B)alkanes and alkynes
C)alkenes and alkynes
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
36
What is the term for a giant molecule composed of small molecules bonded together repeatedly in a long,continuous chain?
A)monomer
B)polymer
C)saturated hydrocarbon
D)unsaturated hydrocarbon
E)none of the above
A)monomer
B)polymer
C)saturated hydrocarbon
D)unsaturated hydrocarbon
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
37
What is the general term for a hydrocarbon that contains only single bonds?
A)aromatic hydrocarbon
B)hydrocarbon derivative
C)saturated hydrocarbon
D)unsaturated hydrocarbon
E)none of the above
A)aromatic hydrocarbon
B)hydrocarbon derivative
C)saturated hydrocarbon
D)unsaturated hydrocarbon
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following is the condensed structural formula for nonane?
A)CH₃-CH₂-CH₂-CH₂-CH₂-CH₂-CH₃
B)CH₃-CH₂-CH₂-CH₂-CH₂-CH₂-CH₂-CH₃
C)CH₃-CH₂-CH₂-CH₂-CH₂-CH₂-CH₂-CH₂-CH₃
D)CH₃-CH₂-CH₂-CH₂-CH₂-CH₂-CH₂-CH₂-CH₂-CH₃
E)none of the above
A)CH₃-CH₂-CH₂-CH₂-CH₂-CH₂-CH₃
B)CH₃-CH₂-CH₂-CH₂-CH₂-CH₂-CH₂-CH₃
C)CH₃-CH₂-CH₂-CH₂-CH₂-CH₂-CH₂-CH₂-CH₃
D)CH₃-CH₂-CH₂-CH₂-CH₂-CH₂-CH₂-CH₂-CH₂-CH₃
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
39
What is the IUPAC name for: CH₃-CH₂-CH₂-CH₃?
A)butane
B)heptane
C)hexane
D)pentane
E)none of the above
A)butane
B)heptane
C)hexane
D)pentane
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
40
What is the term for a chemical formula that shows the arrangement of atoms in a molecule?
A)atomic formula
B)bonding formula
C)molecular formula
D)structural formula
E)none of the above
A)atomic formula
B)bonding formula
C)molecular formula
D)structural formula
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
41
What is the name of the following alkyl group: CH₃-CH₂-CH₂-?
A)ethyl
B)isopropyl
C)methyl
D)propyl
E)none of the above
A)ethyl
B)isopropyl
C)methyl
D)propyl
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
42
What are the two products from the complete combustion of an alkane?
A)CO and H₂
B)CO and H₂O
C)CO₂ and H₂
D)CO₂ and H₂O
E)none of the above
A)CO and H₂
B)CO and H₂O
C)CO₂ and H₂
D)CO₂ and H₂O
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
43
How many isomers share the molecular formula C₆H14?
A)2
B)3
C)4
D)5
E)none of the above
A)2
B)3
C)4
D)5
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
44
What is the IUPAC name for the following? 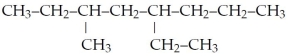
A)isooctane
B)3-ethyl-5-methyloctane
C)4-ethyl-6-methyloctane
D)5-ethyl-3-methyloctane
E)none of the above
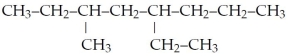
A)isooctane
B)3-ethyl-5-methyloctane
C)4-ethyl-6-methyloctane
D)5-ethyl-3-methyloctane
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
45
How many isomers share the molecular formula C5H₁₂?
A)2
B)3
C)4
D)5
E)none of the above
A)2
B)3
C)4
D)5
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
46
What is the IUPAC name for the following? 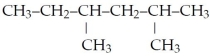
A)octane
B)isohexane
C)2,4-dimethylhexane
D)3,5-dimethylhexane
E)none of the above
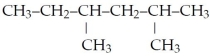
A)octane
B)isohexane
C)2,4-dimethylhexane
D)3,5-dimethylhexane
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
47
What is the IUPAC name for the following? 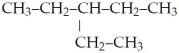
A)heptane
B)isoheptane
C)2-ethylpentane
D)3-ethylpentane
E)none of the above
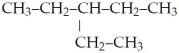
A)heptane
B)isoheptane
C)2-ethylpentane
D)3-ethylpentane
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following is a balanced equation for the complete combustion of ethane?
A)2 C₂H₆ + 5 O₂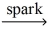 4 CO + 6 H₂O
4 CO + 6 H₂O
B)2 C₂H₆ + 7 O₂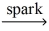 4 CO₂ + 6 H₂O
4 CO₂ + 6 H₂O
C)C₂H₆ + O₂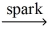 2 CO + 3 H₂
2 CO + 3 H₂
D)C₂H₆ + 2 O₂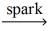 2 CO₂ + 3 H₂
2 CO₂ + 3 H₂
E)C₂H₆ + O₂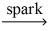 no reaction
no reaction
A)2 C₂H₆ + 5 O₂
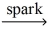 4 CO + 6 H₂O
4 CO + 6 H₂OB)2 C₂H₆ + 7 O₂
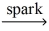 4 CO₂ + 6 H₂O
4 CO₂ + 6 H₂OC)C₂H₆ + O₂
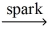 2 CO + 3 H₂
2 CO + 3 H₂D)C₂H₆ + 2 O₂
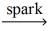 2 CO₂ + 3 H₂
2 CO₂ + 3 H₂E)C₂H₆ + O₂
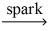 no reaction
no reaction
Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following is a balanced equation for the complete combustion of propane?
A)2 C₃H₈ + 3 O₂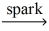 6 CO + 8 H₂
6 CO + 8 H₂
B)2 C₃H₈ + 7 O₂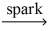 6 CO + 8 H₂O
6 CO + 8 H₂O
C)C₃H₈ + 3 O₂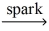 3 CO₂ + 4 H₂
3 CO₂ + 4 H₂
D)C₃H₈ + 5 O₂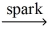 3 CO₂ + 4 H₂O
3 CO₂ + 4 H₂O
E)C₃H₈ + O₂ no reaction
no reaction
A)2 C₃H₈ + 3 O₂
 6 CO + 8 H₂
6 CO + 8 H₂B)2 C₃H₈ + 7 O₂
 6 CO + 8 H₂O
6 CO + 8 H₂OC)C₃H₈ + 3 O₂
 3 CO₂ + 4 H₂
3 CO₂ + 4 H₂D)C₃H₈ + 5 O₂
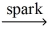 3 CO₂ + 4 H₂O
3 CO₂ + 4 H₂OE)C₃H₈ + O₂
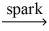 no reaction
no reaction
Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following molecular formulas corresponds to an alkene?
A)C7H₁₂
B)C7H14
C)C7H16
D)all of the above
E)none of the above
A)C7H₁₂
B)C7H14
C)C7H16
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following is a balanced equation for the complete combustion of methane?
A)2 CH₄ + O₂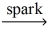 2 CO + 4 H₂
2 CO + 4 H₂
B)2 CH₄ + 3 O₂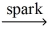 2 CO + 4 H₂O
2 CO + 4 H₂O
C)CH₄ + O₂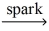 CO₂ + 2 H₂
CO₂ + 2 H₂
D)CH₄ + 2 O₂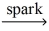 CO₂ + 2 H₂O
CO₂ + 2 H₂O
E)CH₄ + O₂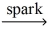 no reaction
no reaction
A)2 CH₄ + O₂
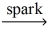 2 CO + 4 H₂
2 CO + 4 H₂B)2 CH₄ + 3 O₂
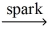 2 CO + 4 H₂O
2 CO + 4 H₂OC)CH₄ + O₂
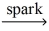 CO₂ + 2 H₂
CO₂ + 2 H₂D)CH₄ + 2 O₂
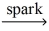 CO₂ + 2 H₂O
CO₂ + 2 H₂OE)CH₄ + O₂
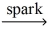 no reaction
no reaction
Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
52
What is the name of the following alkyl group: CH₃-CH₂-?
A)ethyl
B)isopropyl
C)methyl
D)propyl
E)none of the above
A)ethyl
B)isopropyl
C)methyl
D)propyl
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
53
What is the name of the following alkyl group: CH₃-?
A)ethyl
B)isopropyl
C)methyl
D)propyl
E)none of the above
A)ethyl
B)isopropyl
C)methyl
D)propyl
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following molecular formulas corresponds to an alkene?
A)C8H14
B)C8H16
C)C8H18
D)all of the above
E)none of the above
A)C8H14
B)C8H16
C)C8H18
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
55
What is the name of the following alkyl group: (CH₃)2CH-?
A)ethyl
B)isopropyl
C)methyl
D)propyl
E)none of the above
A)ethyl
B)isopropyl
C)methyl
D)propyl
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
56
What is the general molecular formula for the alkene class of compounds?
A)CnH₂n
B)CnH₂n+2
C)CnH₂n-2
D)all of the above
E)none of the above
A)CnH₂n
B)CnH₂n+2
C)CnH₂n-2
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
57
What is the IUPAC name for the following? 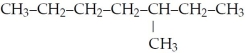
A)octane
B)isooctane
C)3-methylheptane
D)5-methylheptane
E)none of the above
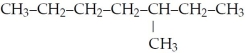
A)octane
B)isooctane
C)3-methylheptane
D)5-methylheptane
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
58
What is the name of the following aryl group: C₆H5-?
A)benzyl
B)hexyl
C)pentyl
D)phenyl
E)none of the above
A)benzyl
B)hexyl
C)pentyl
D)phenyl
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
59
What is the IUPAC name for the following? 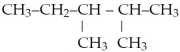
A)heptane
B)isoheptane
C)2-methyl-3-methylpentane
D)2,3-dimethylpentane
E)none of the above
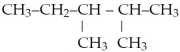
A)heptane
B)isoheptane
C)2-methyl-3-methylpentane
D)2,3-dimethylpentane
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
60
How many isomers share the molecular formula C₃H7F?
A)2
B)3
C)4
D)5
E)none of the above
A)2
B)3
C)4
D)5
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
61
What is the general molecular formula for the alkyne class of compounds?
A)CnH₂n
B)CnH₂n+2
C)CnH₂n-2
D)all of the above
E)none of the above
A)CnH₂n
B)CnH₂n+2
C)CnH₂n-2
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following molecular formulas corresponds to an alkyne?
A)C10H18
B)C10H₂0
C)C10H₂2
D)all of the above
E)none of the above
A)C10H18
B)C10H₂0
C)C10H₂2
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following illustrates the reaction of propene and hydrogen gas in the presence of nickel catalyst at 25 °C and 1 atm?
A)CH₂=CH-CH₃ + H₂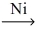 C₃H₄
C₃H₄
B)CH₂=CH-CH₃ + H₂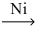 C₃H₆
C₃H₆
C)CH₂=CH-CH₃ + H₂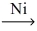 C₃H₈
C₃H₈
D)CH₂=CH-CH₃ + H₂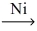 C₆H₆
C₆H₆
E)CH₂=CH-CH₃ + H₂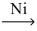 no reaction
no reaction
A)CH₂=CH-CH₃ + H₂
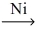 C₃H₄
C₃H₄B)CH₂=CH-CH₃ + H₂
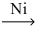 C₃H₆
C₃H₆C)CH₂=CH-CH₃ + H₂
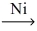 C₃H₈
C₃H₈D)CH₂=CH-CH₃ + H₂
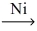 C₆H₆
C₆H₆E)CH₂=CH-CH₃ + H₂
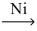 no reaction
no reaction
Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
64
What are the products from the complete combustion of an alkene?
A)CO and H₂
B)CO and H₂O
C)CO₂ and H₂
D)CO₂ and H₂O
E)none of the above
A)CO and H₂
B)CO and H₂O
C)CO₂ and H₂
D)CO₂ and H₂O
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
65
What is the IUPAC name for CH₃-CH=CH-CH₂-CH₂-CH₃?
A)hexene
B)1-hexene
C)2-hexene
D)3-hexene
E)none of the above
A)hexene
B)1-hexene
C)2-hexene
D)3-hexene
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following is the condensed structural formula for 4-nonene?
A)CH₃-CH₂-CH=CH-CH₂-CH₂-CH₂-CH₃
B)CH₃-CH₂-CH₂-CH₂-CH=CH-CH₂-CH₃
C)CH₃-CH₂-CH=CH-CH₂-CH₂-CH₂-CH₂-CH₃
D)CH₃-CH₂-CH₂-CH₂-CH=CH-CH₂-CH₂-CH₃
E)none of the above
A)CH₃-CH₂-CH=CH-CH₂-CH₂-CH₂-CH₃
B)CH₃-CH₂-CH₂-CH₂-CH=CH-CH₂-CH₃
C)CH₃-CH₂-CH=CH-CH₂-CH₂-CH₂-CH₂-CH₃
D)CH₃-CH₂-CH₂-CH₂-CH=CH-CH₂-CH₂-CH₃
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following is the condensed structural formula for 3-heptene?
A)CH₃-CH=CH-CH₂-CH₂-CH₃
B)CH₃-CH₂-CH=CH-CH₂-CH₃
C)CH₃-CH=CH-CH₂-CH₂-CH₂-CH₃
D)CH₃-CH₂-CH=CH-CH₂-CH₂-CH₃
E)none of the above
A)CH₃-CH=CH-CH₂-CH₂-CH₃
B)CH₃-CH₂-CH=CH-CH₂-CH₃
C)CH₃-CH=CH-CH₂-CH₂-CH₂-CH₃
D)CH₃-CH₂-CH=CH-CH₂-CH₂-CH₃
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following pair of alkynes has the same physical properties?
A)1-pentyne and 2-pentyne
B)1-pentyne and isopentyne
C)2-pentyne and isopentyne
D)all of the above
E)none of the above
A)1-pentyne and 2-pentyne
B)1-pentyne and isopentyne
C)2-pentyne and isopentyne
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
69
What is the IUPAC name for CH₃-CH₂-CH₂-CH₂-CH=CH-CH₂-CH₃?
A)octene
B)3-octene
C)4-octene
D)5-octene
E)none of the above
A)octene
B)3-octene
C)4-octene
D)5-octene
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
70
What is the common name for HC≡CH?
A)acetylene
B)ethene
C)ethyne
D)ethylene
E)none of the above
A)acetylene
B)ethene
C)ethyne
D)ethylene
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following is the condensed structural formula for 2-pentene?
A)CH₂=CH-CH₂-CH₂-CH₃
B)CH₃-CH₂-CH=CH-CH₃
C)CH₃-CH=CH-CH₂-CH₂-CH₃
D)CH₃-CH₂-CH₂-CH=CH-CH₃
E)none of the above
A)CH₂=CH-CH₂-CH₂-CH₃
B)CH₃-CH₂-CH=CH-CH₃
C)CH₃-CH=CH-CH₂-CH₂-CH₃
D)CH₃-CH₂-CH₂-CH=CH-CH₃
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following pair of alkenes has the same physical properties?
A)1-butene and 2-butene
B)1-butene and isobutene
C)2-butene and isobutene
D)all of the above
E)none of the above
A)1-butene and 2-butene
B)1-butene and isobutene
C)2-butene and isobutene
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following molecular formulas corresponds to an alkyne?
A)C9H16
B)C9H18
C)C9H₂0
D)all of the above
E)none of the above
A)C9H16
B)C9H18
C)C9H₂0
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
74
What is the IUPAC name for CH₃-CH₂-CH=CH₂?
A)butene
B)1-butene
C)2-butene
D)3-butene
E)none of the above
A)butene
B)1-butene
C)2-butene
D)3-butene
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
75
What is the IUPAC name for CH₃-CH₂-CH₂-CH₂-C≡C-CH₂-CH₃?
A)octyne
B)3-octyne
C)4-octyne
D)5-octyne
E)none of the above
A)octyne
B)3-octyne
C)4-octyne
D)5-octyne
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
76
What is the IUPAC name for CH₃-CH₂-C≡CH?
A)butyne
B)1-butyne
C)2-butyne
D)3-butyne
E)none of the above
A)butyne
B)1-butyne
C)2-butyne
D)3-butyne
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following is the condensed structural formula for 2-heptyne?
A)CH₃-C≡C-CH₂-CH₂-CH₃
B)CH₃-CH₂-C≡C-CH₂-CH₃
C)CH₃-C≡C-CH₂-CH₂-CH₂-CH₃
D)CH₃-CH₂-C≡C-CH₂-CH₂-CH₃
E)none of the above
A)CH₃-C≡C-CH₂-CH₂-CH₃
B)CH₃-CH₂-C≡C-CH₂-CH₃
C)CH₃-C≡C-CH₂-CH₂-CH₂-CH₃
D)CH₃-CH₂-C≡C-CH₂-CH₂-CH₃
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following is the condensed structural formula for 3-nonyne?
A)CH₃-CH₂-C≡C-CH₂-CH₂-CH₂-CH₃
B)CH₃-CH₂-CH₂-CH₂-C≡C-CH₂-CH₃
C)CH₃-CH₂-C≡C-CH₂-CH₂-CH₂-CH₂-CH₃
D)CH₃-CH₂-CH₂-CH₂-C≡C-CH₂-CH₂-CH₃
E)none of the above
A)CH₃-CH₂-C≡C-CH₂-CH₂-CH₂-CH₃
B)CH₃-CH₂-CH₂-CH₂-C≡C-CH₂-CH₃
C)CH₃-CH₂-C≡C-CH₂-CH₂-CH₂-CH₂-CH₃
D)CH₃-CH₂-CH₂-CH₂-C≡C-CH₂-CH₂-CH₃
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following is the condensed structural formula for 1-pentyne?
A)CH≡C-CH₂-CH₂-CH₃
B)CH₃-CH₂-C≡C-CH₃
C)CH₃-C≡C-CH₂-CH₂-CH₃
D)CH₃-CH₂-CH₂-C≡C-CH₃
E)none of the above
A)CH≡C-CH₂-CH₂-CH₃
B)CH₃-CH₂-C≡C-CH₃
C)CH₃-C≡C-CH₂-CH₂-CH₃
D)CH₃-CH₂-CH₂-C≡C-CH₃
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
80
What is the IUPAC name for CH₃-C≡C-CH₂-CH₂-CH₃?
A)hexyne
B)1-hexyne
C)2-hexyne
D)3-hexyne
E)none of the above
A)hexyne
B)1-hexyne
C)2-hexyne
D)3-hexyne
E)none of the above

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck



