Deck 3: The Evolution of Atomic Theory
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/88
Play
Full screen (f)
Deck 3: The Evolution of Atomic Theory
1
Identify X in the expression 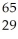 X.
X.
A) selenium
B) copper
C) cobalt
D) cesium
E) manganese
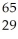 X.
X.A) selenium
B) copper
C) cobalt
D) cesium
E) manganese
copper
2
A 3.45 gram sample of hematite (Fe2O₃) that contains 2.41 grams of Fe has what percentage Fe by mass?
A) 41.0%
B) 31.1%
C) 69.9%
D) 59.9%
A) 41.0%
B) 31.1%
C) 69.9%
D) 59.9%
69.9%
3
Identify X in the expression 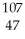 X.
X.
A) silver
B) gold
C) bohrium
D) cobalt
E) neodymium
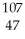 X.
X.A) silver
B) gold
C) bohrium
D) cobalt
E) neodymium
silver
4
An atomic mass unit, also known as amu, is equal to ________ the mass of a 12C atom.
A) 1/2
B) 1/12
C) 1/6
D) 1/1840
A) 1/2
B) 1/12
C) 1/6
D) 1/1840

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
5
How is the term "mass number" defined for a given atom or isotope?
A) as the mass of one mole of that element or isotope
B) as the sum of the number of protons, neutrons, and electrons
C) as the sum of the number of protons and neutrons in the nucleus
D) as the number of protons in the nucleus
A) as the mass of one mole of that element or isotope
B) as the sum of the number of protons, neutrons, and electrons
C) as the sum of the number of protons and neutrons in the nucleus
D) as the number of protons in the nucleus

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
6
What is the relative charge on a neutron, proton, and electron?
A) 0, -1, +1
B) 0, 0, -1
C) 0, +1, -1
D) -1, +1, -1
A) 0, -1, +1
B) 0, 0, -1
C) 0, +1, -1
D) -1, +1, -1

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
7
A neutral atom's atomic number is equal to ________.
A) the number of nucleons
B) the sum of the number of protons and neutrons
C) the number of electrons
D) the number of neutrons
A) the number of nucleons
B) the sum of the number of protons and neutrons
C) the number of electrons
D) the number of neutrons

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
8
Calcium carbonate (CaCO₃) is 34.5% Ca by mass. In a 418 g sample of CaCO₃, how many grams of calcium are present?
A) 144 g
B) 40.08 g
C) 235.1 g
D) 83.6 g
A) 144 g
B) 40.08 g
C) 235.1 g
D) 83.6 g

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
9
Identify X in the expression 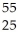 X.
X.
A) zinc
B) iron
C) iridium
D) cesium
E) manganese
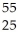 X.
X.A) zinc
B) iron
C) iridium
D) cesium
E) manganese

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
10
The breakthrough Dmitri Mendeleev had when constructing the periodic table occurred ________.
A) when J. J. Thomson discovered the electron
B) when Sir James Chadwick discovered the neutron
C) when Thomson and E. Goldstein discovered the proton
D) when Mendeleev placed the elements in a sequence of increasing atomic mass
A) when J. J. Thomson discovered the electron
B) when Sir James Chadwick discovered the neutron
C) when Thomson and E. Goldstein discovered the proton
D) when Mendeleev placed the elements in a sequence of increasing atomic mass

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
11
What is the mass of the single product that is formed from the complete reaction of 2.5 g hydrogen with 7.5 g oxygen?
A) 5.0 g
B) 7.5 g
C) 9.5 g
D) 10.0 g
A) 5.0 g
B) 7.5 g
C) 9.5 g
D) 10.0 g

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
12
How is the term "atomic number" defined for a given atom?
A) as the mass of one mole of that element or isotope
B) as the sum of the number of protons, neutrons, and electrons
C) as the sum of the number of protons and neutrons in the nucleus
D) as the number of protons in the nucleus
A) as the mass of one mole of that element or isotope
B) as the sum of the number of protons, neutrons, and electrons
C) as the sum of the number of protons and neutrons in the nucleus
D) as the number of protons in the nucleus

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
13
How many protons does the nucleus of the sulfur-35 isotope have?
A) 16
B) 19
C) 32
D) 35
A) 16
B) 19
C) 32
D) 35

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
14
A compound is produced when 6.08 g of nitrogen combine with 13.92 g oxygen. What is the empirical formula of this compound?
A) NO
B) NO₂
C) N₂O₃
D) N₂O5
A) NO
B) NO₂
C) N₂O₃
D) N₂O5

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
15
24.3 g of magnesium yield 40.3 g MgO upon combustion with oxygen. How much magnesium needs to be burned to yield 12.1 g MgO?
A) 4.30 g
B) 6.10 g
C) 7.30 g
D) 8.40 g
A) 4.30 g
B) 6.10 g
C) 7.30 g
D) 8.40 g

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
16
How many neutrons does the nucleus of the phosphorus-32 isotope have?
A) 15
B) 16
C) 17
D) 32
A) 15
B) 16
C) 17
D) 32

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
17
What is the atomic number of uranium-235?
A) 235
B) 238
C) 92
D) 143
A) 235
B) 238
C) 92
D) 143

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
18
According to the experiments of Ernest Rutherford, where can a majority of a given atom's mass be found?
A) scattered randomly throughout an approximately cube-shaped atom
B) at the center of an approximately spherical atom
C) in concentric layers of spherical shape
D) as small pieces orbiting a common center in circular paths
A) scattered randomly throughout an approximately cube-shaped atom
B) at the center of an approximately spherical atom
C) in concentric layers of spherical shape
D) as small pieces orbiting a common center in circular paths

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is the correct symbolic representation of the lead-209 isotope?
A)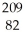 Li
Li
B)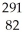 Pb
Pb
C)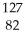 Pb
Pb
D)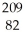 Pb
Pb
A)
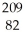 Li
LiB)
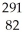 Pb
PbC)
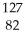 Pb
PbD)
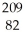 Pb
Pb
Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
20
The structure of individual atoms can roughly be described as ________.
A) positively charged center and mostly empty space
B) an even mixture of mass and charge scattered throughout a spherical shape
C) positively charged, low-mass pieces in orbit around a massive, negatively charged center
D) positively charged pieces in orbit around a low-mass, negatively charged center
A) positively charged center and mostly empty space
B) an even mixture of mass and charge scattered throughout a spherical shape
C) positively charged, low-mass pieces in orbit around a massive, negatively charged center
D) positively charged pieces in orbit around a low-mass, negatively charged center

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following elements is a halogen?
A) He
B) B
C) F
D) Kr
A) He
B) B
C) F
D) Kr

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
22
Which is expected to be the most chemically similar element to phosphorus?
A) S
B) As
C) Se
D) O
A) S
B) As
C) Se
D) O

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following is not a main group element?
A) S
B) As
C) Cu
D) Xe
A) S
B) As
C) Cu
D) Xe

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following elements can have an anion with 18 electrons?
A) Cl
B) Ar
C) Sc
D) Fe
A) Cl
B) Ar
C) Sc
D) Fe

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following is a transition metal in the fourth period of the periodic table?
A) Ga
B) Ca
C) Fe
D) Ag
A) Ga
B) Ca
C) Fe
D) Ag

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
26
How many electrons are found in a Fe₃+ ion?
A) 23
B) 26
C) 29
D) 56
A) 23
B) 26
C) 29
D) 56

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following is a transition element?
A) Co
B) Eu
C) Es
D) He
A) Co
B) Eu
C) Es
D) He

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following terms applies to the horizontal rows found on a periodic table?
A) arrangement
B) group
C) period
D) isotope
A) arrangement
B) group
C) period
D) isotope

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
29
What name is commonly given to the elements of Group IIA of the periodic table?
A) alkaline earth metals
B) transition metals
C) platinum metals
D) alkali metals
A) alkaline earth metals
B) transition metals
C) platinum metals
D) alkali metals

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
30
Which ion contains 18 electrons?
A) Ca+
B) CL₂-
C) Ar-
D) P3-
A) Ca+
B) CL₂-
C) Ar-
D) P3-

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following is not a metal?
A) Ca
B) Pt
C) Kr
D) Mg
A) Ca
B) Pt
C) Kr
D) Mg

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following is a metal?
A) Xe
B) C
C) As
D) W
A) Xe
B) C
C) As
D) W

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following terms applies to the vertical columns found on a periodic table?
A) arrangement
B) group
C) period
D) isotope
A) arrangement
B) group
C) period
D) isotope

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
34
Which representative element is found in the fourth group and fifth period?
A) Br
B) Te
C) Sn
D) Ge
A) Br
B) Te
C) Sn
D) Ge

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
35
What name is commonly given to the elements of Group IA of the periodic table?
A) alkaline earth metals
B) transition metals
C) platinum metals
D) alkali metals
A) alkaline earth metals
B) transition metals
C) platinum metals
D) alkali metals

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following elements is a chalcogen?
A) H
B) N
C) O
D) F
A) H
B) N
C) O
D) F

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following elements is an actinide?
A) Np
B) Pr
C) W
D) Fr
A) Np
B) Pr
C) W
D) Fr

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following is a metalloid in the third period of the periodic table?
A) Al
B) Si
C) P
D) S
A) Al
B) Si
C) P
D) S

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
39
Which element would have a similar chemical reactivity in water as K?
A) Ca
B) Ar
C) Rb
D) H
A) Ca
B) Ar
C) Rb
D) H

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following elements may have a cation that has 16 electrons?
A) Si
B) P
C) S
D) Cl
A) Si
B) P
C) S
D) Cl

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following pairs are isotopes?
A) "Ca₂+ and Mg2+"
B) "199Hg and 200Hg"
C) "18O and 19F"
D) "238U and 238Np"
A) "Ca₂+ and Mg2+"
B) "199Hg and 200Hg"
C) "18O and 19F"
D) "238U and 238Np"

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
42
The law of constant composition states that atoms must combine in a one to one ratio.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
43
Which has more electrons?
A) Br-
B) Sr 2+
C) Rb
D) As 3+
A) Br-
B) Sr 2+
C) Rb
D) As 3+

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following has the highest ionization energy?
A) O
B) Sn
C) As
D) C
A) O
B) Sn
C) As
D) C

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
45
Which element, isotope, or ion listed below contains 16 protons, 17 neutrons, and 18 electrons?
A) "33Ar2-"
B) "33S2-"
C) "33Cl"
D) "34S2-"
A) "33Ar2-"
B) "33S2-"
C) "33Cl"
D) "34S2-"

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
46
Which cation has the atomic number of 20 and a charge of +2?
A) C₂+
B) Mg2+
C) Ca₂+
D) S2+
A) C₂+
B) Mg2+
C) Ca₂+
D) S2+

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following has more electrons than neutrons?
A) 24Mg2+
B) 27Al
C) 75As3-
D) 32S2-
A) 24Mg2+
B) 27Al
C) 75As3-
D) 32S2-

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
48
In Dalton's atomic theory, all matter is made up of atoms.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
49
An alpha particle consists of two protons and two neutrons.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
50
How many protons are found in a N3- ion?
A) 7
B) 10
C) 14
D) 15
A) 7
B) 10
C) 14
D) 15

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
51
The atomic weight for an atom is the weighted average of all the naturally occurring isotopes.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
52
Ionization energy ________ from top to bottom in a group and ________ from left to right in a period.
A) increases; increases
B) increases; decreases
C) decreases; increases
D) decreases; decreases
A) increases; increases
B) increases; decreases
C) decreases; increases
D) decreases; decreases

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
53
Which element, isotope, or ion listed below contains 35 protons, 46 neutrons, and 36 electrons?
A) "81Kr+"
B) "81Br"
C) "81Kr"
D) "81Br -"
A) "81Kr+"
B) "81Br"
C) "81Kr"
D) "81Br -"

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
54
The conservation of mass law states that matter can neither be created nor destroyed during a chemical reaction.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
55
Fr+ is called ________.
A) a cation
B) an anion
C) an isotope
D) a non-metal
A) a cation
B) an anion
C) an isotope
D) a non-metal

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
56
Which species has 16 protons, 18 neutrons and 18 electrons?
A) "34S2+"
B) "34S2-"
C) "36Ar2+"
D) "36Se2-"
A) "34S2+"
B) "34S2-"
C) "36Ar2+"
D) "36Se2-"

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
57
Atoms combine to form molecules (compounds) in simple, whole number ratios.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
58
Ions are ________.
A) atoms that have gained electrons
B) atoms or groups of atoms with a net electrical charge that is not equal to zero
C) atoms that have lost electrons
D) all of the above
A) atoms that have gained electrons
B) atoms or groups of atoms with a net electrical charge that is not equal to zero
C) atoms that have lost electrons
D) all of the above

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following has more protons than electrons?
A) Mg2+
B) Al
C) P3-
D) O₂-
A) Mg2+
B) Al
C) P3-
D) O₂-

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following has the lowest ionization energy?
A) F
B) P
C) Ca
D) Cs
A) F
B) P
C) Ca
D) Cs

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
61
Platinum is used in the catalytic converters of the cars.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
62
Krypton is a noble gas.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
63
Iodine is the only non-metal that is a solid at room temperature.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
64
The periodic table contains more elements that are classified as non-metals than elements of any other type.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
65
Each element is defined by its atomic number.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
66
Group VIIIA of the periodic table contains elements that are gaseous non-metals.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
67
A neutral atom of the isotope phosphorus-32 contains 15 protons, 17 neutrons, and 15 electrons.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
68
Sulfur belongs to the chalcogen family.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
69
The oxygen-17 isotope has more protons than neutrons in its nucleus.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
70
Two isotopes of the same element differ only in the number of neutrons found in the nucleus.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
71
Magnesium is the element that appears in the second group of the second period.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
72
Lanthanides are rare earth elements.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
73
Earth's gravitational force is incapable of keeping the atmospheric helium from escaping into space.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
74
Radium is the only noble gas that is radioactive.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
75
Barium is an alkali earth metal.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
76
Tritium is an isotope of carbon.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
77
Noble gases occupy Group VII in the periodic table.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
78
The oxygen-17 (-2) isotopic anion contains more electrons than either protons or neutrons.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
79
Group IIA of the periodic table contains elements that are gaseous non-metals.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
80
Mercury is the only metal that is a liquid at room temperature.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck



