Deck 12: Solutions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/143
Play
Full screen (f)
Deck 12: Solutions
1
Which of the following statements is correct?
A) Energy is needed to overcome the solute-solute attractive forces.
B) Energy is needed to overcome the solvent-solvent attractive forces.
C) Solvation releases energy.
D) All the above statements are correct.
A) Energy is needed to overcome the solute-solute attractive forces.
B) Energy is needed to overcome the solvent-solvent attractive forces.
C) Solvation releases energy.
D) All the above statements are correct.
All the above statements are correct.
2
Solutions are considered to be which general type of matter?
A) homogeneous mixtures
B) heterogeneous mixtures
C) pure substances
D) pure elements
A) homogeneous mixtures
B) heterogeneous mixtures
C) pure substances
D) pure elements
homogeneous mixtures
3
Which of the following is not a solution?
A) ice in water
B) vinegar in water
C) sugar in water
D) metal alloys
A) ice in water
B) vinegar in water
C) sugar in water
D) metal alloys
ice in water
4
How are the solubilities of gases (solutes) in liquids (solvents) affected by changing pressure?
A) Gases are more soluble in liquids when the pressure is decreased.
B) Pressure has no effect on how soluble a gas is in a liquid.
C) Gases are less soluble in liquids when the pressure is increased.
D) Gases are more soluble in liquids when the pressure is increased.
A) Gases are more soluble in liquids when the pressure is decreased.
B) Pressure has no effect on how soluble a gas is in a liquid.
C) Gases are less soluble in liquids when the pressure is increased.
D) Gases are more soluble in liquids when the pressure is increased.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
5
If a solute is to be soluble in a given solvent, which step in the solution-making process must have a negative value for its energy?
A) the physical separation of solvent particles
B) the physical separation of solute particles
C) the formation of solvent/solute interactions
D) All three of the preceding steps must be negative for a solute to be soluble.
A) the physical separation of solvent particles
B) the physical separation of solute particles
C) the formation of solvent/solute interactions
D) All three of the preceding steps must be negative for a solute to be soluble.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is a solution?
A) air
B) amalgam
C) metal alloys
D) All of the above are solutions.
A) air
B) amalgam
C) metal alloys
D) All of the above are solutions.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following has the lowest hydration energy?
A) sodium chloride
B) magnesium chloride
C) aluminum chloride
D) silicon tetrachloride
A) sodium chloride
B) magnesium chloride
C) aluminum chloride
D) silicon tetrachloride

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
8
In a solution made by dissolving 50 grams of calcium chloride ( CaCl2 ) in 1000 grams of water, which component is considered to be the solute?
A) Water is the solute.
B) Both water and calcium chloride are solutes in this example.
C) Calcium chloride is the solute.
D) The solute cannot be determined from the information given.
A) Water is the solute.
B) Both water and calcium chloride are solutes in this example.
C) Calcium chloride is the solute.
D) The solute cannot be determined from the information given.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
9
Under what conditions would a gas be most soluble in a liquid?
A) high temperature and high pressure
B) high temperature and low pressure
C) low temperature and high pressure
D) low temperature and low pressure
A) high temperature and high pressure
B) high temperature and low pressure
C) low temperature and high pressure
D) low temperature and low pressure

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
10
How are solubility and temperature generally related in a system consisting of a solid solute and a liquid solvent?
A) Solubility generally increases with increasing temperature.
B) Solubility generally increases with decreasing temperature.
C) Changing the temperature has no effect on solubility.
D) Solubility generally decreases with increasing temperature.
A) Solubility generally increases with increasing temperature.
B) Solubility generally increases with decreasing temperature.
C) Changing the temperature has no effect on solubility.
D) Solubility generally decreases with increasing temperature.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
11
In a dilute saline solution the solute is in the form of ________.
A) Na+ and Cl-
B) NaCl (s)
C) NaCl (ℓ)
D) both A and B
A) Na+ and Cl-
B) NaCl (s)
C) NaCl (ℓ)
D) both A and B

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
12
In a solution made by dissolving 200 grams of sugar in 1000 grams of water, which component is considered to be the solvent?
A) Water is the solvent.
B) Both water and sugar are solvents in this example.
C) Sugar is the solvent.
D) The solvent cannot be determined from the information given.
A) Water is the solvent.
B) Both water and sugar are solvents in this example.
C) Sugar is the solvent.
D) The solvent cannot be determined from the information given.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
13
Sterling silver is a solution (ALLOY) of ________.
A) silver and zinc
B) silver and gold
C) silver and copper
D) silver and iron
A) silver and zinc
B) silver and gold
C) silver and copper
D) silver and iron

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following examples demonstrates a net decrease in entropy?
A) melting an ice cube
B) adding cream to hot coffee
C) ripping a finished jigsaw puzzle into a thousand pieces
D) assembling a 1000-piece jigsaw puzzle
E) All of the preceding are examples of net increases in entropy.
A) melting an ice cube
B) adding cream to hot coffee
C) ripping a finished jigsaw puzzle into a thousand pieces
D) assembling a 1000-piece jigsaw puzzle
E) All of the preceding are examples of net increases in entropy.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following has the highest hydration energy?
A) sodium chloride
B) magnesium chloride
C) aluminum chloride
D) silicon tetrachloride
A) sodium chloride
B) magnesium chloride
C) aluminum chloride
D) silicon tetrachloride

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
16
The statement "like dissolves like" justifies why ________.
A) water will dissolve ethyl alcohol
B) octane will dissolve methane
C) water will not dissolve fats
D) All of the above statements are correct.
A) water will dissolve ethyl alcohol
B) octane will dissolve methane
C) water will not dissolve fats
D) All of the above statements are correct.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
17
The solute in a saline solution is ________.
A) water
B) salt
C) the salt solution
D) One cannot say unless the exact concentration is known.
A) water
B) salt
C) the salt solution
D) One cannot say unless the exact concentration is known.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following statements is correct?
A) Temperature usually increases the solubility of a solid in water.
B) Pressure usually increases the solubility of a gas in water.
C) Solubility involves the statement "like dissolves like."
D) All of the above statements are correct.
A) Temperature usually increases the solubility of a solid in water.
B) Pressure usually increases the solubility of a gas in water.
C) Solubility involves the statement "like dissolves like."
D) All of the above statements are correct.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following solutions are possible?
A) Both the solvent and solute are liquids.
B) Both the solvent and solute are solids.
C) The solvent is a liquid and the solute is a gas.
D) All of them are possible.
A) Both the solvent and solute are liquids.
B) Both the solvent and solute are solids.
C) The solvent is a liquid and the solute is a gas.
D) All of them are possible.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following is the least water soluble at 50°C?
A) Ce2(SO₄)3
B) KBr
C) NaNO₃
D) CH₃COONa
A) Ce2(SO₄)3
B) KBr
C) NaNO₃
D) CH₃COONa

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
21
200. mL of a 0.100 M NaCl solution contains ________ g of NaCl.
A) 1.17
B) 11.7
C) 117
D) 5.85
A) 1.17
B) 11.7
C) 117
D) 5.85

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
22
25 mL of a 0.1 M NaCl solution contains ________ g of NaCl.
A) 0.0025
B) 0.15
C) 2.5
D) 0.25
A) 0.0025
B) 0.15
C) 2.5
D) 0.25

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
23
The spherical structure of a soap molecule that is formed when soap molecules are in contact with water is called ________.
A) detergent
B) hydrophobic end
C) hydrophilic end
D) micelle
A) detergent
B) hydrophobic end
C) hydrophilic end
D) micelle

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
24
10.0 mL of 0.50 M hydrochloric acid neutralizes ________ mL of 0.50 M magnesium hydroxide solution.
A) 5.0
B) 10.
C) 20.
D) 40.
A) 5.0
B) 10.
C) 20.
D) 40.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
25
What is the molarity of 3.68 mL of a solution containing 0.0025 moles of calcium chloride?
A) 6.8 × 10-4 M
B) 0.68 M
C) 1.5 M
D) 6.8 × 10-3 M
A) 6.8 × 10-4 M
B) 0.68 M
C) 1.5 M
D) 6.8 × 10-3 M

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
26
500 mL of a 0.4 M potassium dichromate contains ________ moles of potassium ions and ________ moles of dichromate ions.
A) 0.4; 0.2
B) 0.2; 0.4
C) 0.4; 0.6
D) 0.2; 0.2
A) 0.4; 0.2
B) 0.2; 0.4
C) 0.4; 0.6
D) 0.2; 0.2

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
27
Soap consists of a long ________ head and a long ________ tail.
A) polar; polar
B) polar; nonpolar
C) nonpolar; polar
D) nonpolar; nonpolar
A) polar; polar
B) polar; nonpolar
C) nonpolar; polar
D) nonpolar; nonpolar

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
28
Which end, the polar end or the nonpolar end, of a detergent molecule is attracted to water?
A) the polar end
B) both the polar and the nonpolar end
C) neither the polar nor the nonpolar end
D) the nonpolar end
A) the polar end
B) both the polar and the nonpolar end
C) neither the polar nor the nonpolar end
D) the nonpolar end

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following is the most water soluble at 50°C?
A) CH₃COONa
B) NaNO₃
C) Ce2(SO₄)3
D) KBr
A) CH₃COONa
B) NaNO₃
C) Ce2(SO₄)3
D) KBr

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
30
10.0 mL of 0.50 M sulfuric acid neutralizes ________ mL of 0.50 M ammonium hydroxide solution.
A) 5.0
B) 10.
C) 20.
D) 40.
A) 5.0
B) 10.
C) 20.
D) 40.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
31
Which end, the polar end or the nonpolar end, of a detergent molecule is attracted to dirt particles?
A) the polar end
B) both the polar and the nonpolar end
C) neither the polar nor the nonpolar end
D) the nonpolar end
A) the polar end
B) both the polar and the nonpolar end
C) neither the polar nor the nonpolar end
D) the nonpolar end

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
32
500 mL 0.4 M aluminum nitrate solution contains ________ moles of aluminum ions and ________ moles of nitrate ions.
A) 0.6; 0.2
B) 0.2; 0.4
C) 0.2; 0.6
D) 0.2; 0.2
A) 0.6; 0.2
B) 0.2; 0.4
C) 0.2; 0.6
D) 0.2; 0.2

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
33
The molarity of a solution that contains 5.85 g of NaCl in 2.0 L of solution is ________.
A) 2.93 M
B) 0.050 M
C) 5.0 M
D) 0.29 M
A) 2.93 M
B) 0.050 M
C) 5.0 M
D) 0.29 M

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
34
500 mL of a 0.4 M sodium nitrate contains ________ moles of sodium ions and ________ moles of nitrate ions.
A) 0.4; 0.2
B) 0.2; 0.4
C) 0.4; 0.6
D) 0.2; 0.2
A) 0.4; 0.2
B) 0.2; 0.4
C) 0.4; 0.6
D) 0.2; 0.2

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
35
7.5 g of calcium carbonate in 150 mL of aqueous solution will provide a ________ M solution.
A) 0.10
B) 0.50
C) 1.0
D) 2.0
A) 0.10
B) 0.50
C) 1.0
D) 2.0

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
36
10.0 mL of 0.50 M HCl solution neutralizes ________ mL of 0.50 M NaOH solution.
A) 5.0
B) 10.
C) 20.
D) 40.
A) 5.0
B) 10.
C) 20.
D) 40.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
37
To prepare 500. mL of 0.5 M NaCl from a stock of 2.0 M NaCl one needs to ________.
A) dilute 125 mL 2.0 M NaCl with enough water to a total of 500. mL solution
B) dilute 125 mL 2.0 M NaCl with 500. mL water
C) dilute 375 mL 2.0 M NaCl with enough water to a total of 500. mL solution
D) dilute 375 mL 2.0 M NaCl with 500. mL water
A) dilute 125 mL 2.0 M NaCl with enough water to a total of 500. mL solution
B) dilute 125 mL 2.0 M NaCl with 500. mL water
C) dilute 375 mL 2.0 M NaCl with enough water to a total of 500. mL solution
D) dilute 375 mL 2.0 M NaCl with 500. mL water

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
38
To prepare 100 mL of 0.4 M HCl solution from a stock solution of 2 M HCl solution one needs to ________.
A) dilute 20 mL of 2 M HCl solution with 100 mL of HCl solution.
B) dilute 20 mL of 2 M HCl solution with enough water to make a total volume of 100 mL of solution.
C) use 500 mL of 2 M HCl solution and boil it down to a final volume of 100 mL of solution.
D) dilute 80 mL of 2 M HCl solution to a total volume of 100 mL of solution.
A) dilute 20 mL of 2 M HCl solution with 100 mL of HCl solution.
B) dilute 20 mL of 2 M HCl solution with enough water to make a total volume of 100 mL of solution.
C) use 500 mL of 2 M HCl solution and boil it down to a final volume of 100 mL of solution.
D) dilute 80 mL of 2 M HCl solution to a total volume of 100 mL of solution.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
39
10.0 mL of 0.50 M sulfuric acid neutralizes ________ mL of 0.50 M magnesium hydroxide solution.
A) 5.0
B) 10.
C) 20.
D) 40.
A) 5.0
B) 10.
C) 20.
D) 40.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
40
500 mL of a 0.4 M calcium nitrite contains ________ moles of calcium ions and ________ moles of nitrite ions.
A) 0.4; 0.2
B) 0.2; 0.4
C) 0.4; 0.6
D) 0.2; 0.2
A) 0.4; 0.2
B) 0.2; 0.4
C) 0.4; 0.6
D) 0.2; 0.2

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
41
A solid solution is a solution of two metals that are not necessarily evenly distributed in each other.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
42
A 2.00 g sample of a non-electrolyte (molar mass = 118) is dissolved in 55.0 g of benzene (Kf of benzene is 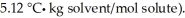 What is the freezing point depression of the solution?
What is the freezing point depression of the solution?
A) 1.58 °C
B) 3.16 °C
C) 0.79 °C
D) 2.33 °C
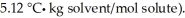 What is the freezing point depression of the solution?
What is the freezing point depression of the solution?A) 1.58 °C
B) 3.16 °C
C) 0.79 °C
D) 2.33 °C

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
43
Transparent solutions are examples of homogeneous matter.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
44
An impurity ________ the boiling point of a compound.
A) decreases
B) increases
C) has no effect on
D) One cannot say unless the physical properties of the compound are known.
A) decreases
B) increases
C) has no effect on
D) One cannot say unless the physical properties of the compound are known.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
45
Vinegar is a liquid solution where acetic acid serves as the solvent.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
46
If the empirical formula is C₄H₃N and the molecular weight is 130 g/mole, then the molecular formula is ________.
A) C16H₁₂N₄
B) C12H9N3
C) C₄H₃N
D) C₈H₆N₂
A) C16H₁₂N₄
B) C12H9N3
C) C₄H₃N
D) C₈H₆N₂

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
47
Phenolphthalein turns ________ when the solution is basic.
A) clear
B) blue
C) pink
D) orange
A) clear
B) blue
C) pink
D) orange

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
48
An impurity ________ the melting point of a compound.
A) decreases
B) increases
C) has no effect on
D) One cannot say unless the physical properties of the compound are known.
A) decreases
B) increases
C) has no effect on
D) One cannot say unless the physical properties of the compound are known.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
49
4.173 g of an organic compound (non-electrolyte) lowered the freezing point of 100.0 g of nitrobenzene by 2.6 °C. If the Kf of nitrobenzene is 8.1 °C∙ kg solvent/mol solute, the molecular mass of the non-electrolyte is ________.
A) 260
B) 195
C) 65
D) 130
A) 260
B) 195
C) 65
D) 130

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following is not a colligative property?
A) molarity
B) freezing point depression
C) vapor pressure lowering
D) boiling point elevation
A) molarity
B) freezing point depression
C) vapor pressure lowering
D) boiling point elevation

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
51
A 0.2119 g sample of an acid, HA, was neutralized by 34.7 mL of 0.0500 M NaOH solution. The molecular mass of the acid is ________.
A) 61
B) 92
C) 122
D) 244
A) 61
B) 92
C) 122
D) 244

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
52
How many grams of NaCl are in 200 g of a 10 percent-by-mass salt solution?
A) 10 g
B) 20 g
C) 40 g
D) 80 g
A) 10 g
B) 20 g
C) 40 g
D) 80 g

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
53
Alloys are solid solutions.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
54
The solvent in a saline solution is water.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
55
The percent composition of a solution made by dissolving 5.0 g NaOH in 20. g of water is ________.
A) 4.0%
B) 5.0%
C) 20.%
D) 25%
A) 4.0%
B) 5.0%
C) 20.%
D) 25%

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
56
A 2.154 g sample of an unknown non-electrolyte is dissolved in 50. g of water (Kf of water = 1.86 °C∙ kg solvent/mol solute). If the solution freezes at -1.23 °C, the molecular mass of the non-electrolyte is ________.
A) 33
B) 65
C) 130
D) 260
A) 33
B) 65
C) 130
D) 260

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
57
The solute in a sugar solution is water.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
58
Henry's law gives the relationship between ________.
A) colligative properties and the freezing point
B) solubility of a gas and concentration
C) solubility of a gas and pressure
D) hydrophobic and hydrophilic solutions
A) colligative properties and the freezing point
B) solubility of a gas and concentration
C) solubility of a gas and pressure
D) hydrophobic and hydrophilic solutions

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
59
How many grams of water are in 100 g of a 30 percent-by-mass sugar solution?
A) 30 g
B) 60 g
C) 70 g
D) 100 g
A) 30 g
B) 60 g
C) 70 g
D) 100 g

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
60
What is the freezing point depression of a sugar solution prepared by dissolving 375.00 g of sucrose (342.3 g/mol) in 1500.0 g of water (Kf of water = 1.86 °C∙ kg solvent/mol solute)?
A) 1.36 °C
B) -1.36 °C
C) -1.86 °C
D) -0.465 °C
A) 1.36 °C
B) -1.36 °C
C) -1.86 °C
D) -0.465 °C

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
61
The bends, a condition encountered by divers, is attributed to the rapid depressurized escape of nitrogen from blood to form bubbles in the blood.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
62
Dry cleaning uses perchloroethane to remove grease and oil from clothes.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
63
A mixture of sand, sodium carbonate, and calcium oxide is a homogeneous solution.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
64
If a liquid is volatile it evaporates readily.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
65
The process of surrounding solute ions with solvent molecules is called solvation.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
66
Situations of great disorder are said to have "high entropies."

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
67
Air is a gas solution where oxygen is the solvent.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
68
For most molecular solids an increase in temperature generally causes an increase in solubility.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
69
It is not possible to make a solution in which both the solute and the solvent are gases.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
70
A hydrophobic group will be attracted to water.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
71
Nonpolar solutes will usually dissolve in polar solvents.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
72
C₈H₁₈ will dissolve in CCl4.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
73
NH₃ will dissolve in BF3.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
74
A mixture is not necessarily a solution.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
75
Once the temperature of 100.°C is reached, liquid water spontaneously becomes a gas.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
76
Polar solutes will usually dissolve in polar solvents.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
77
A solution prepared by dissolving 24 g potassium sulfate in 150 mL total solution has a molarity of 0.92 M.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
78
Molarity is defined as moles of solute per liter of solvent.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
79
Ca2+ is expected to have a greater energy of hydration than K+.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
80
Hydrocarbons, such as pentane C₅H₁₂, are water soluble.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck



