Deck 12: Synthesis
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/95
Play
Full screen (f)
Deck 12: Synthesis
1
Provide the major product(s) obtained from the following reaction: 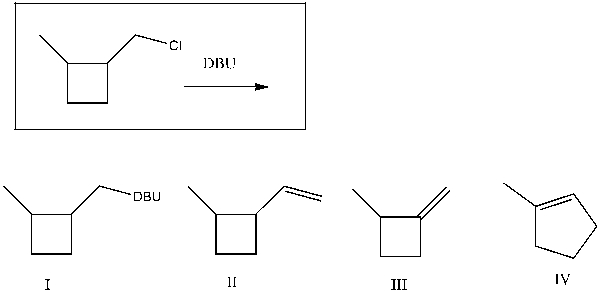
A) I
B) II
C) III
D) IV
E) II and IV

A) I
B) II
C) III
D) IV
E) II and IV
III
2
For the transformation shown, select the most appropriate reagent(s) to effect the change. 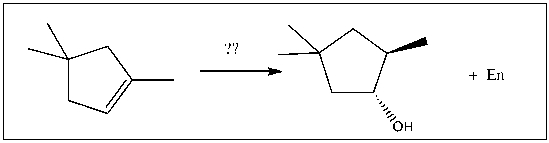
A) 1) OsO4; 2) NaHSO3, H2O
B) 1) Hg(OAc)2, H2O; 2) NaBH4
C) 1) RCO3H; 2) H3O+
D) 1) BH3∙THF; 2) H2O2, NaOH
E) 1) O3; 2) DMS

A) 1) OsO4; 2) NaHSO3, H2O
B) 1) Hg(OAc)2, H2O; 2) NaBH4
C) 1) RCO3H; 2) H3O+
D) 1) BH3∙THF; 2) H2O2, NaOH
E) 1) O3; 2) DMS
1) BH3∙THF; 2) H2O2, NaOH
3
For the transformation shown, select the most appropriate reagent(s) to effect the change. 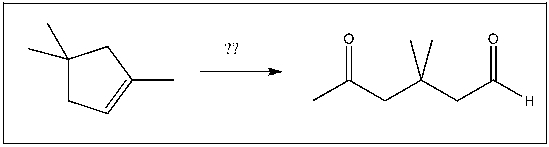
A) 1) OsO4; 2) NaHSO3, H2O
B) 1) Hg(OAc)2, H2O; 2) NaBH4
C) 1) RCO3H; 2) H3O+
D) H2SO4, H2O
E) 1) O3; 2) DMS

A) 1) OsO4; 2) NaHSO3, H2O
B) 1) Hg(OAc)2, H2O; 2) NaBH4
C) 1) RCO3H; 2) H3O+
D) H2SO4, H2O
E) 1) O3; 2) DMS
1) O3; 2) DMS
4
For the transformation shown, select the most appropriate reagent(s) to effect the change. 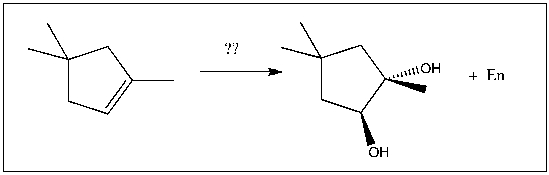
A) 1) OsO4; 2) NaHSO3, H2O
B) 1) Hg(OAc)2, H2O; 2) NaBH4
C) 1) RCO3H; 2) H3O+
D) H2SO4, H2O
E) 1) O3; 2) DMS

A) 1) OsO4; 2) NaHSO3, H2O
B) 1) Hg(OAc)2, H2O; 2) NaBH4
C) 1) RCO3H; 2) H3O+
D) H2SO4, H2O
E) 1) O3; 2) DMS

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
5
Provide the major product(s) for the following transformation: 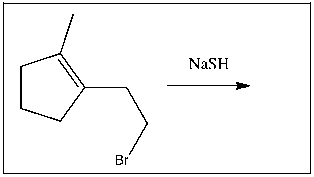
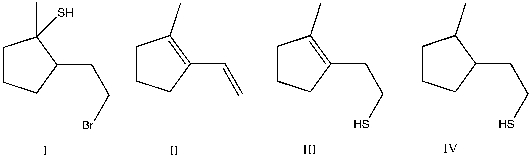
A) I
B) II
C) III
D) IV
E) I and III


A) I
B) II
C) III
D) IV
E) I and III

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
6
For the transformation shown, select the most appropriate reagent(s) to effect the change. 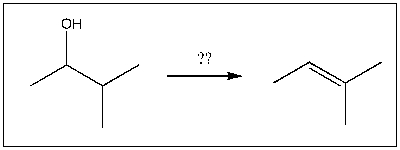
A) NaOH
B) heat/H2SO4
C) CH3CO2H
D) NaOEt
E) KOtBu

A) NaOH
B) heat/H2SO4
C) CH3CO2H
D) NaOEt
E) KOtBu

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
7
For the transformation shown, select the most appropriate reagent(s) to effect the change. 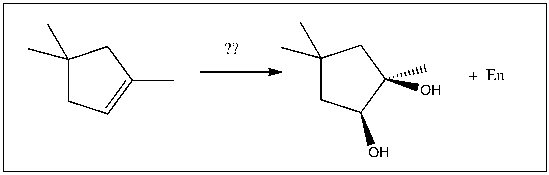
A) 1) OsO4; 2) NaHSO3, H2O
B) 1) Hg(OAc)2, H2O; 2) NaBH4
C) 1) RCO3H; 2) H3O+
D) H2SO4, H2O
E) 1) O3; 2) DMS

A) 1) OsO4; 2) NaHSO3, H2O
B) 1) Hg(OAc)2, H2O; 2) NaBH4
C) 1) RCO3H; 2) H3O+
D) H2SO4, H2O
E) 1) O3; 2) DMS

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
8
Predict the major product(s) for the following reaction: 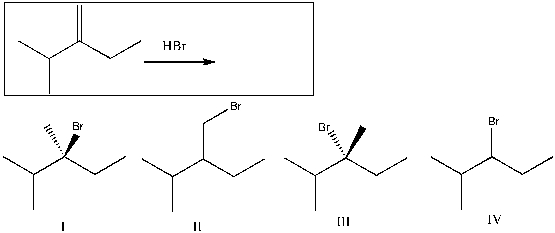
A) I
B) II
C) III
D) IV
E) I and III

A) I
B) II
C) III
D) IV
E) I and III

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
9
For the transformation shown, select the most appropriate reagent(s) to effect the change. 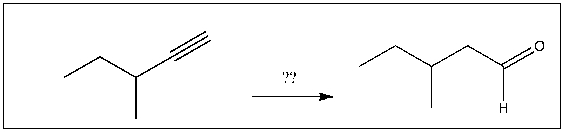
A) 1) OsO4; 2) NaHSO3, H2O
B) 1) Hg(OAc)2, H2O; 2) NaBH4
C) H2, Pt
D) 1) 9-BBN; 2) H2O2, NaOH
E) 1) O3; 2) DMS

A) 1) OsO4; 2) NaHSO3, H2O
B) 1) Hg(OAc)2, H2O; 2) NaBH4
C) H2, Pt
D) 1) 9-BBN; 2) H2O2, NaOH
E) 1) O3; 2) DMS

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
10
For the transformation shown, select the most appropriate reagent(s) to effect the change. 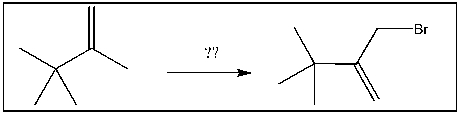
A) HBr
B) Br2/h
C) Br2/ROOR
D) HBr/ROOR
E) h /NBS

A) HBr
B) Br2/h
C) Br2/ROOR
D) HBr/ROOR
E) h /NBS

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
11
For the transformation shown, select the most appropriate reagent(s) to effect the change. 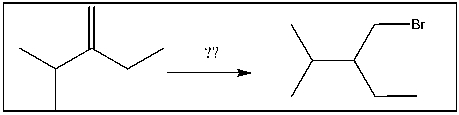
A) HBr
B) Br2/h
C) Br2/ROOR
D) HBr/ROOR
E) h /NBS

A) HBr
B) Br2/h
C) Br2/ROOR
D) HBr/ROOR
E) h /NBS

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
12
For the transformation shown, select the most appropriate reagent(s) to effect the change. 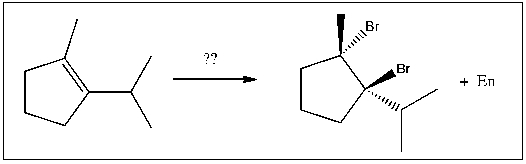
A) HBr
B) Br2/h
C) Br2
D) HBr/ROOR
E) h /NBS

A) HBr
B) Br2/h
C) Br2
D) HBr/ROOR
E) h /NBS

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
13
For the transformation shown, select the most appropriate reagent(s) to effect the change. 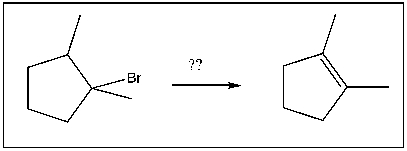
A) NaOtBu
B) HBr
C) H2SO4
D) NaOH
E) NaSH

A) NaOtBu
B) HBr
C) H2SO4
D) NaOH
E) NaSH

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
14
For the transformation shown, select the most appropriate reagent(s) to effect the change. 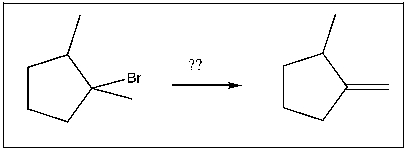
A) NaOtBu
B) HBr
C) H2SO4
D) NaOH
E) NaSH

A) NaOtBu
B) HBr
C) H2SO4
D) NaOH
E) NaSH

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
15
For the transformation shown, select the most appropriate reagent(s) to effect the change. 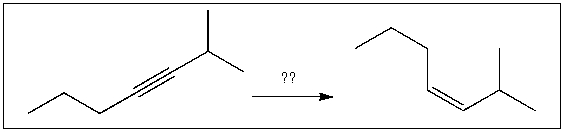
A) 1) OsO4; 2) NaHSO3, H2O
B) NaNH2
C) H2, Pt
D) Na, NH3(l)
E) H2, Lindlar's catalyst

A) 1) OsO4; 2) NaHSO3, H2O
B) NaNH2
C) H2, Pt
D) Na, NH3(l)
E) H2, Lindlar's catalyst

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
16
For the transformation shown, select the most appropriate reagent(s) to effect the change. 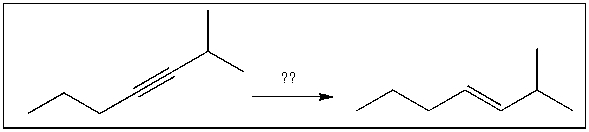
A) 1) OsO4; 2) NaHSO3, H2O
B) NaNH2
C) H2, Pt
D) Na, NH3(l)
E) H2, Lindlar's catalyst

A) 1) OsO4; 2) NaHSO3, H2O
B) NaNH2
C) H2, Pt
D) Na, NH3(l)
E) H2, Lindlar's catalyst

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
17
For the transformation shown, select the most appropriate reagent(s) to effect the change. 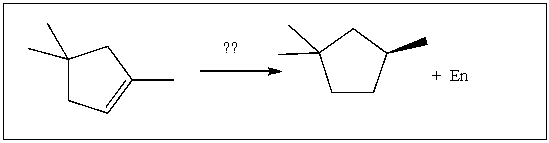
A) 1) OsO4; 2) NaHSO3, H2O
B) 1) Hg(OAc)2, H2O; 2) NaBH4
C) H2, Pt
D) 1) BH3∙THF; 2) H2O2, NaOH
E) 1) O3; 2) DMS

A) 1) OsO4; 2) NaHSO3, H2O
B) 1) Hg(OAc)2, H2O; 2) NaBH4
C) H2, Pt
D) 1) BH3∙THF; 2) H2O2, NaOH
E) 1) O3; 2) DMS

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
18
For the transformation shown, select the most appropriate reagent(s) to effect the change. 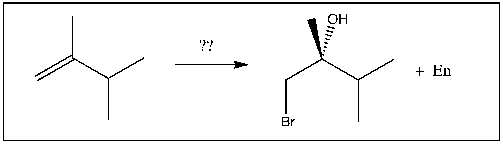
A) HBr
B) Br2/H2O
C) Br2
D) HBr/ROOR
E) h /NBS

A) HBr
B) Br2/H2O
C) Br2
D) HBr/ROOR
E) h /NBS

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
19
For the transformation shown, select the most appropriate reagent(s) to effect the change. 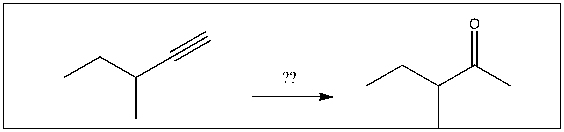
A) 1) OsO4; 2) NaHSO3, H2O
B) H2SO4, H2O, HgSO4
C) H2, Pt
D) 1) 9-BBN; 2) H2O2, NaOH
E) 1) O3; 2) DMS

A) 1) OsO4; 2) NaHSO3, H2O
B) H2SO4, H2O, HgSO4
C) H2, Pt
D) 1) 9-BBN; 2) H2O2, NaOH
E) 1) O3; 2) DMS

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
20
For the transformation shown, select the most appropriate reagent(s) to effect the change. 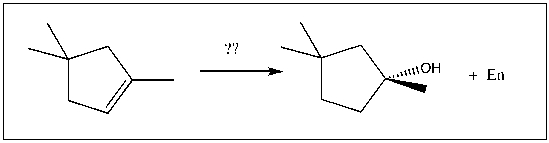
A) 1) OsO4; 2) NaHSO3, H2O
B) 1) Hg(OAc)2, H2O; 2) NaBH4
C) 1) RCO3H; 2) H3O+
D) 1) BH3-THF; 2) H2O2, NaOH
E) 1) O3; 2) DMS

A) 1) OsO4; 2) NaHSO3, H2O
B) 1) Hg(OAc)2, H2O; 2) NaBH4
C) 1) RCO3H; 2) H3O+
D) 1) BH3-THF; 2) H2O2, NaOH
E) 1) O3; 2) DMS

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
21
Which sequence of reagents will accomplish the following transformation? 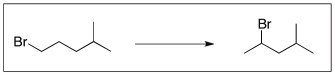
A) 1) KOtBu; 2) HBr
B) 1) NaOEt; 2) HBr, ROOR
C) 1) H2SO4, heat; 2) Br2, h
D) 1) NaOEt; 2) HBr
E) both A and D work

A) 1) KOtBu; 2) HBr
B) 1) NaOEt; 2) HBr, ROOR
C) 1) H2SO4, heat; 2) Br2, h
D) 1) NaOEt; 2) HBr
E) both A and D work

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
22
For the transformation shown, select the most appropriate reagent(s) to effect the change. 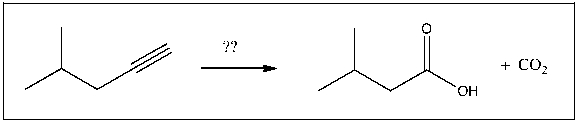
A) 1) OsO4; 2) NaHSO3, H2O
B) H2SO4, H2O, HgSO4
C) NaOH
D) xs NaNH2
E) 1) O3; 2) H2O

A) 1) OsO4; 2) NaHSO3, H2O
B) H2SO4, H2O, HgSO4
C) NaOH
D) xs NaNH2
E) 1) O3; 2) H2O

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
23
Propose an efficient synthesis of propanoic acid from acetylene:

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
24
Which reagents will accomplish the following transformation? 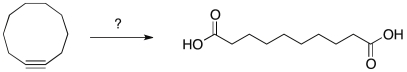
A) NaNH2
B) 1) O3; 2) H2O
C) KMnO4
D) 1) BH3∙THF; 2) H2O2, NaOH
E) H2SO4, H2O

A) NaNH2
B) 1) O3; 2) H2O
C) KMnO4
D) 1) BH3∙THF; 2) H2O2, NaOH
E) H2SO4, H2O

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
25
Compound X has molecular formula C8H10. Reaction of Compound X with excess ozone, followed by reaction with dimethyl sulfide and then washing with water produces only the compounds shown below. Draw a possibility for Compound X that is consistent with these results. 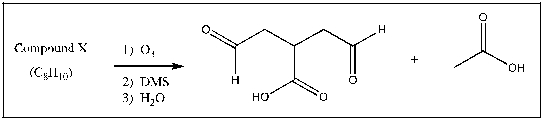


Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following sequences of reagents will move the alcohol functional group from the tertiary position of 1-methyl-1-cyclohexanol to a secondary position?
A) 1) KOtBu; 2) Hg(OAc)2, H2O; 3) NaBH4
B) 1) TsCl, pyr; 2) KOtBu; 3) BH3-THF; 4) H2O2, NaOH
C) 1) H2SO4, heat; 2) BH3∙THF; 3) H2O2, NaOH
D) 1) TsCl, pyr; 2) NaOH; 3) BH3∙THF; 4) H2O2, NaOH
E) C and D will both work
A) 1) KOtBu; 2) Hg(OAc)2, H2O; 3) NaBH4
B) 1) TsCl, pyr; 2) KOtBu; 3) BH3-THF; 4) H2O2, NaOH
C) 1) H2SO4, heat; 2) BH3∙THF; 3) H2O2, NaOH
D) 1) TsCl, pyr; 2) NaOH; 3) BH3∙THF; 4) H2O2, NaOH
E) C and D will both work

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
27
Which sequence of reagents will accomplish the following transformation? 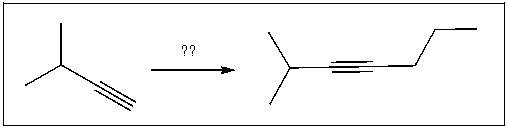
A) 1) KOtBu; 2) 1-bromopropane
B) 1) NaNH2; 2) 1-bromopropane
C) 1) NaNH2; 2) 2-bromopropane
D) 1) O3; 2) DMS
E) 1) O3; 2) 1-bromopropane

A) 1) KOtBu; 2) 1-bromopropane
B) 1) NaNH2; 2) 1-bromopropane
C) 1) NaNH2; 2) 2-bromopropane
D) 1) O3; 2) DMS
E) 1) O3; 2) 1-bromopropane

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
28
Select the best reagent to convert 4,5-dimethylhex-2-yne to 4,5-dimethylhex-1-yne.
A) 1) OsO4; 2) NaHSO3, H2O
B) HBr, ROOR
C) NaBr
D) 1) xs NaNH2; 2) H3O+
E) HBr
A) 1) OsO4; 2) NaHSO3, H2O
B) HBr, ROOR
C) NaBr
D) 1) xs NaNH2; 2) H3O+
E) HBr

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
29
For the transformation shown, select the most appropriate reagent(s) to effect the change. 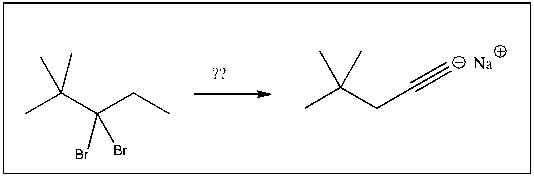
A) 1) OsO4; 2) NaHSO3, H2O
B) H2SO4, H2O, HgSO4
C) NaOH
D) xs NaNH2
E) 1) O3; 2) DMS

A) 1) OsO4; 2) NaHSO3, H2O
B) H2SO4, H2O, HgSO4
C) NaOH
D) xs NaNH2
E) 1) O3; 2) DMS

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
30
Predict a correct sequence of reactants required to complete the following transformation in which propanal is the only carbon-containing product. 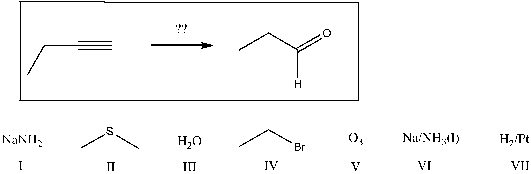
A) 1) I; 2) III; 3) IV; 4) V; 5) II
B) 1) VI; 2) IV; 3) IV; 4) VII; 5) V; 6) II
C) 1) V; 2) II
D) 1) I; 2) IV; 3) VI; 4) V; 5) II
E) none of the above are correct

A) 1) I; 2) III; 3) IV; 4) V; 5) II
B) 1) VI; 2) IV; 3) IV; 4) VII; 5) V; 6) II
C) 1) V; 2) II
D) 1) I; 2) IV; 3) VI; 4) V; 5) II
E) none of the above are correct

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
31
For the transformation shown, select the most appropriate reagent(s) to effect the change. 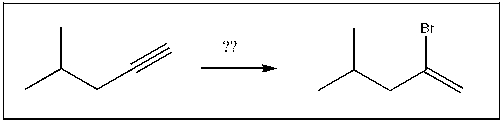
A) 1) OsO4; 2) NaHSO3, H2O
B) HBr, ROOR
C) NaBr
D) xs NaNH2
E) HBr

A) 1) OsO4; 2) NaHSO3, H2O
B) HBr, ROOR
C) NaBr
D) xs NaNH2
E) HBr

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
32
Propose an efficient synthesis of cyclopentanone from 1-methylcyclopentane: 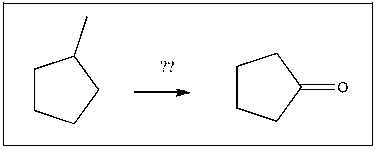


Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
33
Reaction of compound A (molecular formula = C7H12) with sodium amide followed by reaction with 1-bromobutane produces (CH3)2CHCH(CH3)CC(CH2)3CH3. What is the name of compound A?
A) 2,3-dimethylnon-4-yne
B) 2,2-dimethylpent-1-yne
C) 3,4-dimethylpent-1-yne
D) 4,4-dimethylhept-1-yne
E) 1-heptyne
A) 2,3-dimethylnon-4-yne
B) 2,2-dimethylpent-1-yne
C) 3,4-dimethylpent-1-yne
D) 4,4-dimethylhept-1-yne
E) 1-heptyne

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
34
Predict the products of the following reaction: 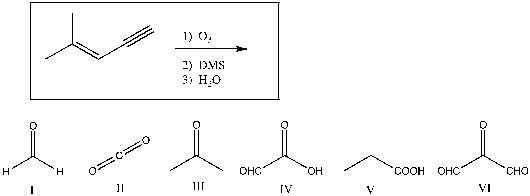
A) I, III, and IV
B) II, III, and IV
C) I, III, and V
D) II, IV, and VI
E) III and IV

A) I, III, and IV
B) II, III, and IV
C) I, III, and V
D) II, IV, and VI
E) III and IV

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
35
Reaction of compound A (molecular formula = C12H24) with ozone followed by reaction with DMS produces only (CH3)3CCH2CHO. Which of the following could be compound A?
A) cis-2,3,7,8-tetramethyloct-4-ene
B) trans-dodec-6-ene
C) 4,4-dimethylpent-1-ene
D) cis-2,2,7,7-tetramethyloct-4-ene
E) 2,2-dimethyldec-4-ene
A) cis-2,3,7,8-tetramethyloct-4-ene
B) trans-dodec-6-ene
C) 4,4-dimethylpent-1-ene
D) cis-2,2,7,7-tetramethyloct-4-ene
E) 2,2-dimethyldec-4-ene

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
36
One compound is produced when acetylene is treated with the following reagents. What is the product? 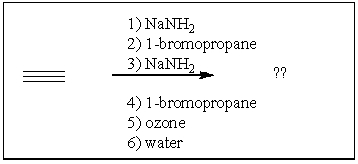


Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
37
Predict the major product(s) for the reaction of pent-1-en-4-yne with sodium amide followed by reaction with bromoethane.
A) Hept-1-en-4-yne
B) Hept-6-en-3-yne
C) Hept-3-en-6-yne
D) Hept-4-en-1-yne
A) Hept-1-en-4-yne
B) Hept-6-en-3-yne
C) Hept-3-en-6-yne
D) Hept-4-en-1-yne

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
38
For the transformation shown, select the most appropriate reagent(s) to effect the change. 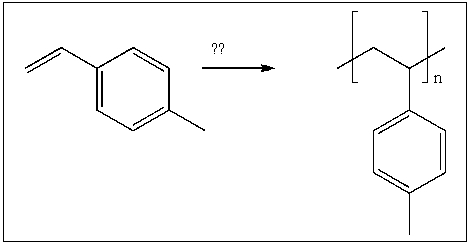
A) 1) OsO4; 2) NaHSO3, H2O
B) ROOR, heat
C) NaOH
D) xs NaNH2
E) 1) O3; 2) H2O

A) 1) OsO4; 2) NaHSO3, H2O
B) ROOR, heat
C) NaOH
D) xs NaNH2
E) 1) O3; 2) H2O

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
39
Select the best reagent to convert 1-bromo-1-methylcyclohexane to 1-bromo-2-methylcyclohexane.
A) 1) KOtBu; 2) HBr
B) 1) NaOEt; 2) HBr
C) 1) NaOEt; 2) HBr, ROOR
D) 1) KOtBu; 2) HBr, ROOR
E) Br2, h
A) 1) KOtBu; 2) HBr
B) 1) NaOEt; 2) HBr
C) 1) NaOEt; 2) HBr, ROOR
D) 1) KOtBu; 2) HBr, ROOR
E) Br2, h

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
40
Predict the major product(s) for the following reaction: 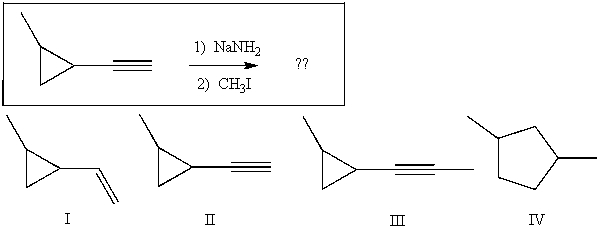
A) I
B) II
C) III
D) IV
E) II and III

A) I
B) II
C) III
D) IV
E) II and III

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following sequences efficiently converts 2-methylpropene and sodium acetylide into 3-methylbutanal? 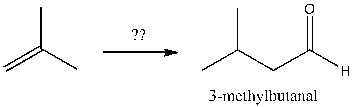
A) 1) HBr; 2) NaCCH; 3) O3; 4) H2O
B) 1) HBr; 2) NaCCH; 3) O3; 4) DMS
C) 1) HBr, ROOR; 2) NaCCH; 3) O3; 4) H2O
D) 1) HBr, ROOR; 2) NaCCH; 3) H2/Ni2B 4) O3; 5) DMS
E) 1) NaCCH; 2) H2/Ni2B; 3) O3; 4) DMS
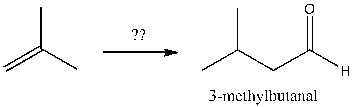
A) 1) HBr; 2) NaCCH; 3) O3; 4) H2O
B) 1) HBr; 2) NaCCH; 3) O3; 4) DMS
C) 1) HBr, ROOR; 2) NaCCH; 3) O3; 4) H2O
D) 1) HBr, ROOR; 2) NaCCH; 3) H2/Ni2B 4) O3; 5) DMS
E) 1) NaCCH; 2) H2/Ni2B; 3) O3; 4) DMS

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
42
Propose an efficient method of converting 3-methyl-2-butanol into 3-methyl-1-butanol.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following provides an efficient method of converting 3-bromo-2-methyl-1-butene into 2-methyl-2-butene?
A) 1) NaOH; 2) H2, Pt
B) 1) H2, Pt; 2) NaOEt
C) 1) H2, Pt; 2) Br2
D) 1) H2, Ni2B; 2) KOtBu
E) 1) H2, Pt; 2) KOtBu
A) 1) NaOH; 2) H2, Pt
B) 1) H2, Pt; 2) NaOEt
C) 1) H2, Pt; 2) Br2
D) 1) H2, Ni2B; 2) KOtBu
E) 1) H2, Pt; 2) KOtBu

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
44
Propose an efficient method of completing the following transformation: 


Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
45
What is the minimum number of steps required to convert 2-methylpropane into 1-bromo-2-methyl-2-propanol?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
46
Using retrosynthetic synthesis, determine which compound(s) could lead to the alcohol shown below in a single step: 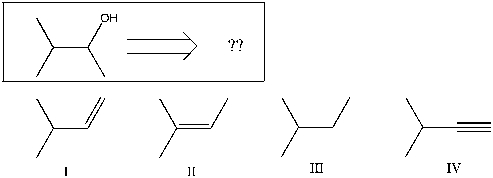
A) I
B) II
C) III
D) IV
E) I or II
) I, II, or IV

A) I
B) II
C) III
D) IV
E) I or II
) I, II, or IV

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
47
Using retrosynthetic analysis, determine which compound(s) could lead to the alkyne shown below in a single step: 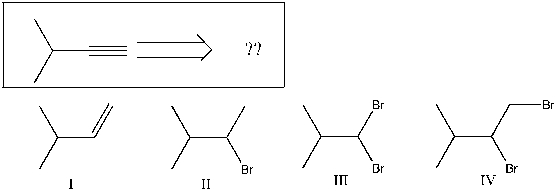
A) I
B) II
C) III
D) IV
E) I or II
) I, II, or IV

A) I
B) II
C) III
D) IV
E) I or II
) I, II, or IV

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
48
Propose an efficient synthesis of 1-butene from propyne:

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
49
Propose a strategy to prepare the target as one of the major products: 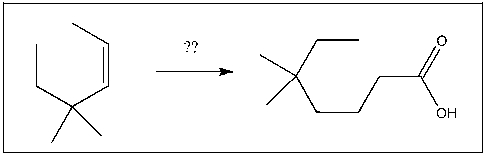


Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
50
Which order of reactions would most effectively transform trans-2-butene into 1-butene?
A) convert to the dibromoalkane, then to the terminal alkyne, then to the terminal alkene
B) convert to an alcohol, then to a terminal alkyne, finally to the terminal alkene
C) convert to the terminal alkene in one step
D) shorten the chain by two carbons, then add a two-carbon alkene to the end
E) convert to an alkane, then to a terminal alkyne, and finally to a terminal alkene
A) convert to the dibromoalkane, then to the terminal alkyne, then to the terminal alkene
B) convert to an alcohol, then to a terminal alkyne, finally to the terminal alkene
C) convert to the terminal alkene in one step
D) shorten the chain by two carbons, then add a two-carbon alkene to the end
E) convert to an alkane, then to a terminal alkyne, and finally to a terminal alkene

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
51
Propose an efficient method of converting 3-methyl-1-butanol into 3-methyl-2-butanol.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following sequences most efficiently converts 3-methyl-1-pentene into 3-bromo-3-methylpentane?
A) 1) Br2; 2) NaOH; 3) HBr
B) 1) Br2, h ; 2) H2, Pt
C) 1) H2, Pt; 2) Br2, h
D) 1) NBS, h ; 2) H2, Pt
E) 1) HBr, ROOR; 2) NaOH; 3) HBr
A) 1) Br2; 2) NaOH; 3) HBr
B) 1) Br2, h ; 2) H2, Pt
C) 1) H2, Pt; 2) Br2, h
D) 1) NBS, h ; 2) H2, Pt
E) 1) HBr, ROOR; 2) NaOH; 3) HBr

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
53
Using retrosynthetic synthesis, determine which compound(s) could lead to the alkene shown below in a single step: 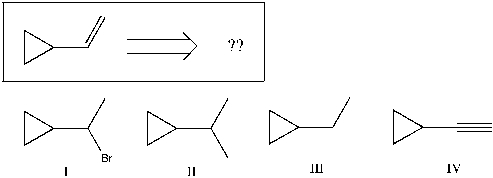
A) I
B) II
C) III
D) IV
E) I or IV
F) I, III, or IV

A) I
B) II
C) III
D) IV
E) I or IV
F) I, III, or IV

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
54
Propose an efficient method of completing the following transformation: 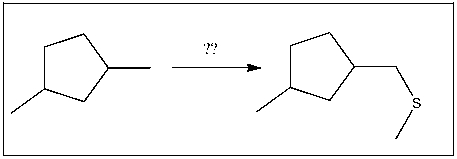


Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
55
Propose an efficient method of completing the following transformation: 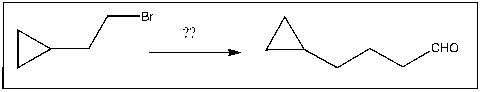


Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
56
Propose an efficient method of completing the following transformation: 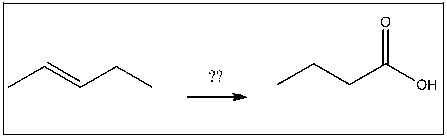


Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following alkenes cannot be converted into an alkyne by reaction with bromine followed by excess sodium amide and then with water? 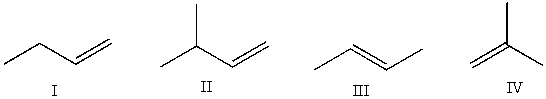
A) I
B) II
C) III
D) IV
E) III and IV

A) I
B) II
C) III
D) IV
E) III and IV

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
58
Identify the changes that must occur in transforming cis-2-butene into 2-butanol:
A) only the identity of the functional group(s) must change
B) only the carbon skeleton must change
C) only the location of the functional group(s) must change
D) only the identity and location of the functional group(s) must change
E) both the carbon skeleton and the identity of the functional group(s) must change
A) only the identity of the functional group(s) must change
B) only the carbon skeleton must change
C) only the location of the functional group(s) must change
D) only the identity and location of the functional group(s) must change
E) both the carbon skeleton and the identity of the functional group(s) must change

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
59
Propose an efficient method of completing the following transformation: 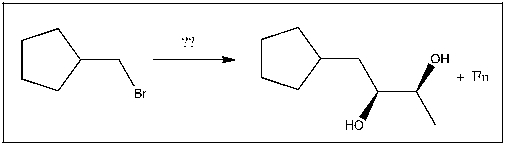


Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
60
What is the minimum number of steps required to convert 2-methylpropane into 2-methylpropene?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following effectively produces 4,4-dimethyl-2-pentyne?
A)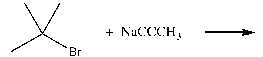
B)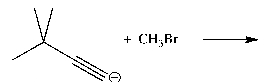
C)
D)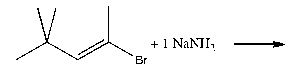
A)
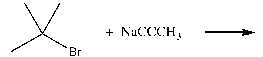
B)
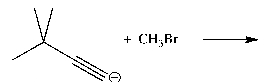
C)

D)


Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
62
Devise a method of converting acetylene into the polymer shown below. 


Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
63
Show that you understand the concept of retrosynthetic analysis by working backwards two steps in the synthesis below. Identify possible combinations of A and B that can lead to the alkyne (C): 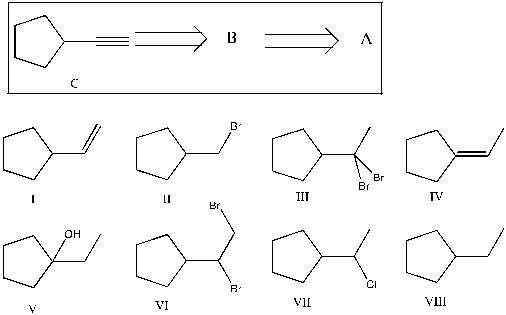
A) B = I and A = VI
B) B = VI and A = I
C) B = III and A = VII
D) B = IV and A = VII
E) B = VI and A = VIII

A) B = I and A = VI
B) B = VI and A = I
C) B = III and A = VII
D) B = IV and A = VII
E) B = VI and A = VIII

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
64
Show that you understand the concept of retrosynthetic analysis by working backwards two steps in the synthesis below. Identify possible combinations of A and B that can lead to the alcohol (C): 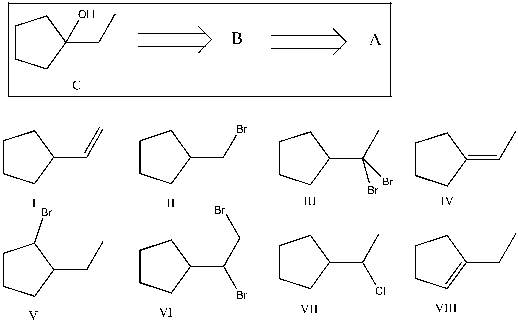
A) B = I and A = VI
B) B = VI and A = I
C) B = III and A = VII
D) B = VII and A = IV
E) B = VIII and A = V

A) B = I and A = VI
B) B = VI and A = I
C) B = III and A = VII
D) B = VII and A = IV
E) B = VIII and A = V

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
65
Show that you understand the concept of retrosynthetic analysis by working backwards two steps in the synthesis below. Identify possible combinations of A and B that can lead to the alkene (C): 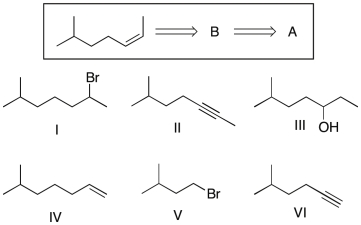
A) B = I and A = IV
B) B = II and A = VI
C) B = III and A = I
D) B = I and A = VI
E) B = III and A = II

A) B = I and A = IV
B) B = II and A = VI
C) B = III and A = I
D) B = I and A = VI
E) B = III and A = II

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
66
Devise an efficient synthesis of the diol shown below starting with 1,1,3,3-tetramethyl-2-ethylcyclohexane. 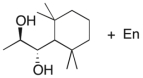


Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
67
Show that you understand the concept of retrosynthetic analysis by working backwards two steps in the synthesis below. Identify possible combinations of A and B that can lead to the compound shown (C): 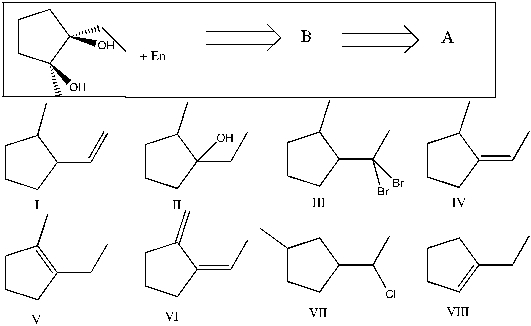
A) B = I and A = VI
B) B = V and A = II
C) B = IV and A = VII
D) B = I and A = III
E) B = VIII and A = V

A) B = I and A = VI
B) B = V and A = II
C) B = IV and A = VII
D) B = I and A = III
E) B = VIII and A = V

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
68
Devise an efficient synthesis of the compound shown below starting with 4-methyl-2-pentanol. 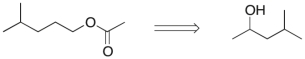
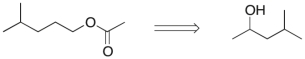

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
69
Devise a method to complete the following synthesis. 


Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
70
Using retrosynthetic analysis, determine which compound(s) could lead to the alkane shown below in a single step: 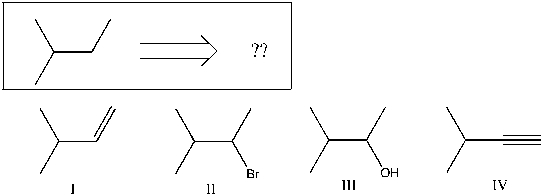
A) II or III
B) I or IV
C) I
D) III
E) IV

A) II or III
B) I or IV
C) I
D) III
E) IV

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
71
Starting with a primary alkyl bromide, which of the following results in an overall increase in the length of the carbon skeleton by one carbon?
A) substitute bromide with acetylide, then cleave the triple bond.
B) substitute bromide with acetylide, then reduce the alkyne to an alkene.
C) substitute bromide with methoxide.
D) eliminate hydrogen bromide to produce an alkene.
A) substitute bromide with acetylide, then cleave the triple bond.
B) substitute bromide with acetylide, then reduce the alkyne to an alkene.
C) substitute bromide with methoxide.
D) eliminate hydrogen bromide to produce an alkene.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
72
Devise an efficient synthesis of the diol shown below starting with 1,1,3,3-tetramethyl-2-ethylcyclohexane. 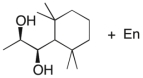


Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
73
Starting with a primary alkyl bromide, which of the following results in an overall decrease in the length of the carbon skeleton by one carbon?
A) substitute bromide with acetylide, then cleave the triple bond.
B) substitute bromide with acetylide, then reduce the alkyne to an alkene.
C) substitute bromide with methoxide.
D) eliminate hydrogen bromide to produce an alkene, then cleave the double bond.
A) substitute bromide with acetylide, then cleave the triple bond.
B) substitute bromide with acetylide, then reduce the alkyne to an alkene.
C) substitute bromide with methoxide.
D) eliminate hydrogen bromide to produce an alkene, then cleave the double bond.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
74
Devise a method to complete the following synthesis. 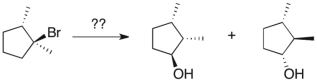


Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
75
Devise a method of converting ethylene into PVC (polyvinyl chloride).

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
76
Devise a method of converting methylcyclobutane into cyclopentene. 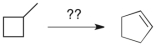


Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
77
Devise a method of converting trans-2-methyl-3-hexene into 5-methylhexanal.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
78
Using retrosynthetic analysis, determine which compound(s) could lead to the bromoalkene shown below in a single step: 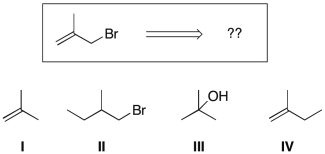
A) I or III
B) I or IV
C) I
D) II
E) III
F) IV

A) I or III
B) I or IV
C) I
D) II
E) III
F) IV

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
79
Show that you understand the concept of retrosynthetic analysis by working backwards two steps in the synthesis below. Identify possible combinations of A and B that could lead to the alkyl halide (C): 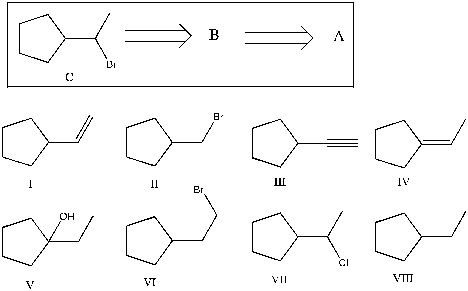
A) B = I and A = VIII
B) B = VI and A = I
C) B = III and A = VII
D) B = IV and A = VII
E) B = V and A = VIII

A) B = I and A = VIII
B) B = VI and A = I
C) B = III and A = VII
D) B = IV and A = VII
E) B = V and A = VIII

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
80
Devise a method of converting trans-2-methyl-3-hexene into 4-methylpentanoic acid.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck



