Deck 1: A Review of General Chemistry: Electrons, Bonds, and Molecular Properties
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/191
Play
Full screen (f)
Deck 1: A Review of General Chemistry: Electrons, Bonds, and Molecular Properties
1
What is the correct Lewis structure for PH3? 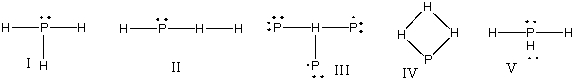
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V
I
2
Which of the following compounds are constitutional isomers of each other? 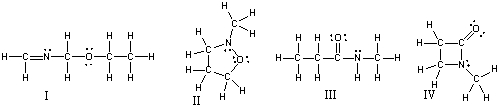
A) I & II
B) III & IV
C) I, II & III
D) I & IV
E) All of these

A) I & II
B) III & IV
C) I, II & III
D) I & IV
E) All of these
I, II & III
3
What is the formal charge on oxygen in the following structure? 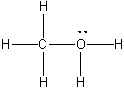
A) 2-
B) 1-
C) 2+
D) 1+
E) 0

A) 2-
B) 1-
C) 2+
D) 1+
E) 0
1+
4
What is the relationship between the following compounds? 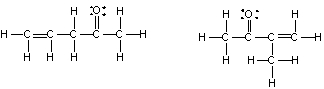
A) They are isotopes
B) They are constitutional isomers
C) They are the same structure
D) They are composed of different elements
E) There is no relationship
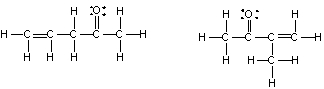
A) They are isotopes
B) They are constitutional isomers
C) They are the same structure
D) They are composed of different elements
E) There is no relationship

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
5
Chemical reactions occur as a result of:
A) Attraction between opposite charges
B) Nucleus-Nucleus interactions
C) Motion of electrons
D) Like atoms interacting
E) Combining two chemicals
A) Attraction between opposite charges
B) Nucleus-Nucleus interactions
C) Motion of electrons
D) Like atoms interacting
E) Combining two chemicals

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
6
What is the correct Lewis dot structure for C? 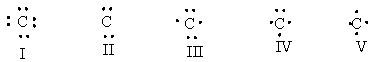
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following compounds are constitutional isomers of each other? 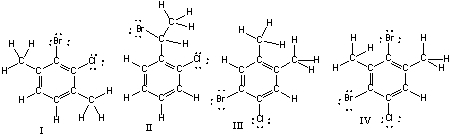
A) I & II
B) III & IV
C) I, II & III
D) II, III & IV
E) All of these

A) I & II
B) III & IV
C) I, II & III
D) II, III & IV
E) All of these

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
8
Constitutional isomers may not differ in what aspects?
A) Physical properties
B) Atomic connectivity
C) Molecular formula
D) Name
E) Constitution
A) Physical properties
B) Atomic connectivity
C) Molecular formula
D) Name
E) Constitution

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
9
Carbon generally forms four bonds and is considered:
A) Tetravalent
B) Divalent
C) Trivalent
D) Monovalent
E) Qudravalent
A) Tetravalent
B) Divalent
C) Trivalent
D) Monovalent
E) Qudravalent

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following compounds are constitutional isomers of each other? 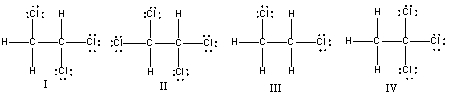
A) I & II
B) III & IV
C) II & III
D) I & IV
E) All of these

A) I & II
B) III & IV
C) II & III
D) I & IV
E) All of these

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
11
What is the correct Lewis structure for CH3CO2H? 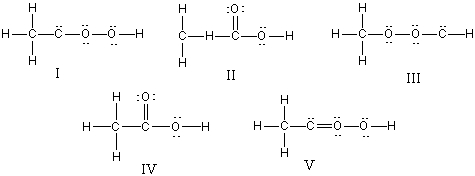
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
12
Credit for the first synthesis of an organic compound from an inorganic precursor is generally ascribed to:
A) Berzelius
B) Arrhenius
C) Kekulé
D) Wöhler
E) Lewis
A) Berzelius
B) Arrhenius
C) Kekulé
D) Wöhler
E) Lewis

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
13
What was long thought to be the difference between inorganic and organic compounds?
A) The number of atoms
B) The synthesis of organic compounds required a vital force
C) The molecular weight
D) Inorganic compounds exhibited a strong nuclear force
E) Inorganic compounds were composed exclusively of transition metals
A) The number of atoms
B) The synthesis of organic compounds required a vital force
C) The molecular weight
D) Inorganic compounds exhibited a strong nuclear force
E) Inorganic compounds were composed exclusively of transition metals

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
14
Draw three constitutional isomers that have molecular formula C4H8BrCl

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
15
Draw three constitutional isomers that have molecular formula C4H8O.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
16
What is the relationship between the following compounds? 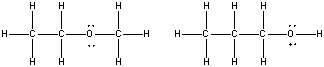
A) Resonance isomers
B) Constitutional isomers
C) Empirical isomers
D) There is no relationship

A) Resonance isomers
B) Constitutional isomers
C) Empirical isomers
D) There is no relationship

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
17
What is the correct Lewis structure for COCl2? 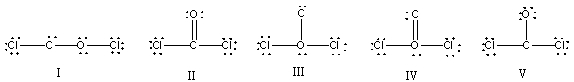
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
18
What is the correct Lewis dot structure for S? 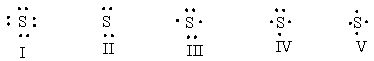
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following compounds has two lone pairs on the central atom?
A) CO2
B) SCl2
C) NF3
D) CS2
E) SO3
A) CO2
B) SCl2
C) NF3
D) CS2
E) SO3

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
20
What force is not taken into account in the formation of a covalent bond?
A) Repulsion between two positively charged nuclei
B) Repulsion between electron clouds on individual atoms
C) Force of attraction between positively charged nuclei and electrons
D) Repulsion of electrons by neutrons
E) All forces listed are involved in forming a covalent bond
A) Repulsion between two positively charged nuclei
B) Repulsion between electron clouds on individual atoms
C) Force of attraction between positively charged nuclei and electrons
D) Repulsion of electrons by neutrons
E) All forces listed are involved in forming a covalent bond

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
21
What is the formal charge on nitrogen in the following structure? 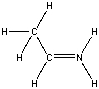
A) 2-
B) 1-
C) 2+
D) 1+
E) 0

A) 2-
B) 1-
C) 2+
D) 1+
E) 0

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
22
What is the correct Lewis structure for hydrocyanic acid (HCN) including the formal charges, if any? 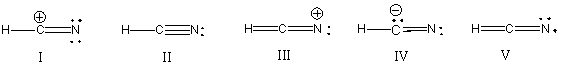
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following structures have 1+ formal charge on the central atom? 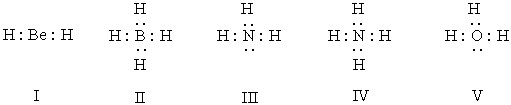
A) I
B) II
C) III
D) III & V
E) IV & V
A) I
B) II
C) III
D) III & V
E) IV & V

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
24
Draw Lewis structure for -CH2CN including formal charges?

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
25
What is the formal charge on oxygen in the following structure? 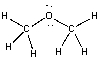
A) 2+
B) 2-
C) 1+
D) 1-
E) 0

A) 2+
B) 2-
C) 1+
D) 1-
E) 0

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
26
What is the formal charge on oxygen in the following structure? 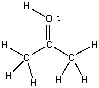
A) 0
B) 1+
C) 2+
D) 1-
E) 2-

A) 0
B) 1+
C) 2+
D) 1-
E) 2-

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
27
The bonding pattern of oxygen with a formal charge of -1 could be described as:
A) One lone pair of electrons and three single bonds
B) Two lone pairs of electrons and two single bonds
C) Three lone pairs of electrons, and one single bond
D) One lone pair of electrons, one single, and one double bond
E) Zero lone pairs, and two single and one double bond
A) One lone pair of electrons and three single bonds
B) Two lone pairs of electrons and two single bonds
C) Three lone pairs of electrons, and one single bond
D) One lone pair of electrons, one single, and one double bond
E) Zero lone pairs, and two single and one double bond

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
28
What is the correct Lewis structure for nitric acid (HNO3) including the formal charges? 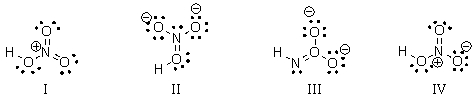
A) I
B) II
C) III
D) IV
E) None of these
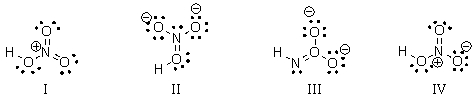
A) I
B) II
C) III
D) IV
E) None of these

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
29
What is the correct Lewis structure for N2O including the formal charges, if any? 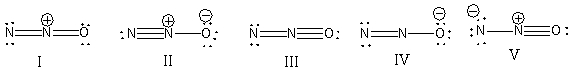
A) I
B) II
C) III
D) IV
E) V
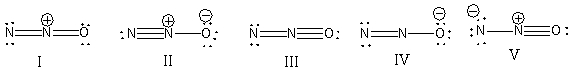
A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
30
What is the correct Lewis structure for hydrazoic acid (HN3) including the formal charges, if any? 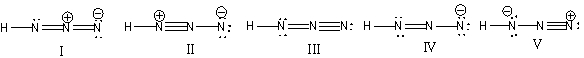
A) I
B) II
C) III
D) IV
E) V
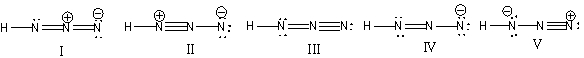
A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
31
What is the correct Lewis structure for SCN- including the formal charges, if any? 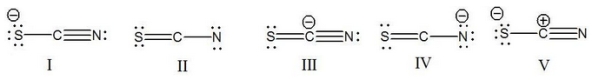
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
32
What are the formal charges on boron and fluorine in the following structure? 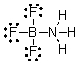
A) B = 1+, N = 1+
B) B = 1+, N = 1-
C) B = 1-, N = 1-
D) B = 1-, N = 1+
E) B = 1-, N = 0
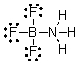
A) B = 1+, N = 1+
B) B = 1+, N = 1-
C) B = 1-, N = 1-
D) B = 1-, N = 1+
E) B = 1-, N = 0

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
33
What are the formal charges on boron and oxygen in the following structure? 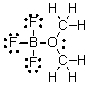
A) B = 1-, O = 1-
B) B = 1-, O = 1+
C) B = 1+, O = 1+
D) B = 1+, O = 1-
E) B = 1-, O = 0

A) B = 1-, O = 1-
B) B = 1-, O = 1+
C) B = 1+, O = 1+
D) B = 1+, O = 1-
E) B = 1-, O = 0

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following structures have zero formal charge on carbon atom? 
A) I & III
B) II & III
C) III & IV
D) I & IV
E) II & IV

A) I & III
B) II & III
C) III & IV
D) I & IV
E) II & IV

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following structures have 1- formal charge on sulfur atom? 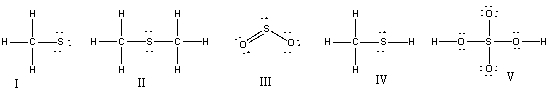
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
36
In ammonium ion, nitrogen has a valence of 4, and zero nonbonding electrons. What is the correct formal charge of nitrogen with 4 covalent bonds?
A) 2-
B) 2+
C) 1-
D) 1+
E) 0
A) 2-
B) 2+
C) 1-
D) 1+
E) 0

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following structures have 1- formal charge on nitrogen atom? 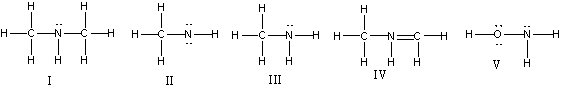
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following structures have 1+ formal charge on sulfur atom? 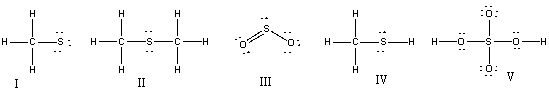
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following structures have formal charge on at least one atom? 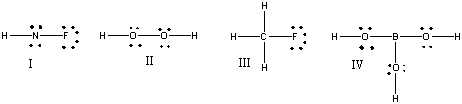
A) I
B) II
C) III
D) IV
E) None of these

A) I
B) II
C) III
D) IV
E) None of these

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
40
Draw Lewis structure for NH2CN including formal charges, if any?

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
41
The Cl-Cl bond is best described as___________.
A) Nonpolar covalent
B) Polar covalent
C) Ionic
D) Coordinate covalent
E) None of these
A) Nonpolar covalent
B) Polar covalent
C) Ionic
D) Coordinate covalent
E) None of these

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following compounds have both polar covalent and ionic bonds?
A) NH4Br
B) H2O2
C) HCN
D) H2S
E) None of these
A) NH4Br
B) H2O2
C) HCN
D) H2S
E) None of these

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following is the most electronegative element?
A) P
B) N
C) S
D) O
E) F
A) P
B) N
C) S
D) O
E) F

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
44
The electronegativity of elements on the periodic table tends to increase_______.
A) From left to right, top to bottom
B) From right to left, bottom to top
C) From left to right, bottom to top
D) From right to left, top to bottom
E) F
A) From left to right, top to bottom
B) From right to left, bottom to top
C) From left to right, bottom to top
D) From right to left, top to bottom
E) F

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following is the least electronegative element?
A) B
B) C
C) N
D) O
E) F
A) B
B) C
C) N
D) O
E) F

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following is the correct representation of dipole for P-Cl bond? 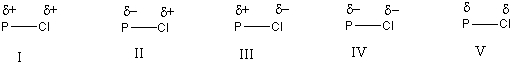
A) I
B) II
C) III
D) IV
E) V
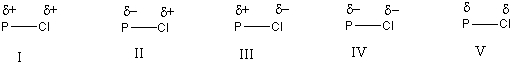
A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
47
Which atom has the most + in the following compound? 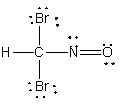
A) N
B) O
C) Br
D) H
E) C

A) N
B) O
C) Br
D) H
E) C

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following is the correct representation of partial charges at the indicated atoms? 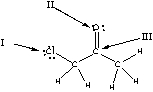
A) I = +; II = +; III = +
B) I = -; II = -; III = -
C) I = +; II = +; III = -
D) I = -; II = -; III = +
E) I = +; II = -; III = +

A) I = +; II = +; III = +
B) I = -; II = -; III = -
C) I = +; II = +; III = -
D) I = -; II = -; III = +
E) I = +; II = -; III = +

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
49
The bond between potassium and oxygen is best described as___________.
A) Nonpolar covalent
B) Polar covalent
C) Ionic
D) Coordinate covalent
E) None of these
A) Nonpolar covalent
B) Polar covalent
C) Ionic
D) Coordinate covalent
E) None of these

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following series has the correct order of elements in increasing electronegativity?
A) C < N < B < Br
B) P < N < As < F
C) Li < B < N < F
D) Cl < Cs < C < Co
E) Be < B < Ba < Br
A) C < N < B < Br
B) P < N < As < F
C) Li < B < N < F
D) Cl < Cs < C < Co
E) Be < B < Ba < Br

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following is the least electronegative element?
A) P
B) N
C) Mg
D) Si
E) K
A) P
B) N
C) Mg
D) Si
E) K

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
52
What is the correct order of increasing electronegativity for Rb, F and O?
A) Rb < F < O
B) Rb < O < F
C) O < F < Rb
D) F < Rb < O
E) None of these
A) Rb < F < O
B) Rb < O < F
C) O < F < Rb
D) F < Rb < O
E) None of these

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
53
For the following compound identify the polar covalent bonds and indicate the direction of dipole moment using + and -. 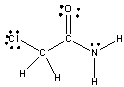


Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
54
For the following compound identify the polar covalent bonds and indicate the direction of dipole moment using + and -. 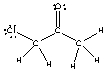


Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following is the most electronegative element?
A) B
B) C
C) N
D) O
E) H
A) B
B) C
C) N
D) O
E) H

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
56
The bond between carbon and hydrogen is best described as___________.
A) Nonpolar covalent
B) Polar covalent
C) Ionic
D) Coordinate covalent
E) None of these
A) Nonpolar covalent
B) Polar covalent
C) Ionic
D) Coordinate covalent
E) None of these

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
57
Draw Lewis structure for ozone (O3,) including formal charges, if any?

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following statements best describes the C-Cl bond in the following compound? 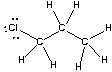
A) nonpolar; no dipole
B) polar; + at carbon and - at chlorine
C) polar; - at carbon and + at chlorine
D) ionic
E) None of these

A) nonpolar; no dipole
B) polar; + at carbon and - at chlorine
C) polar; - at carbon and + at chlorine
D) ionic
E) None of these

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
59
The C-Cl bond is best described as___________.
A) Nonpolar covalent
B) Polar covalent
C) Ionic
D) Coordinate covalent
E) None of these
A) Nonpolar covalent
B) Polar covalent
C) Ionic
D) Coordinate covalent
E) None of these

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following is the correct depiction of induction for a C-F bond? 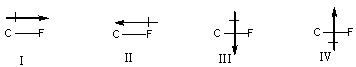
A) I
B) II
C) III
D) IV
E) None of these
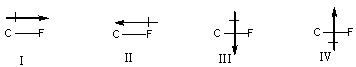
A) I
B) II
C) III
D) IV
E) None of these

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
61
Which element has the electron configuration 1s2 2s2 2p6 3s2 3p5?
A) oxygen
B) fluorine
C) sulfur
D) chlorine
E) bromine
A) oxygen
B) fluorine
C) sulfur
D) chlorine
E) bromine

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
62
For NaSCH3, identify each bond as polar covalent, nonpolar covalent or ionic.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
63
What is the letter designation for the following atomic orbital? 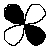
A) s
B) p
C) d
D) f
E) g

A) s
B) p
C) d
D) f
E) g

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
64
In quantum mechanics a node (nodal surface or plane) is:
A) location where is negative
B) location where is positive
C) location where 2 is positive
D) location where 2 is negative
E) location where is zero
A) location where is negative
B) location where is positive
C) location where 2 is positive
D) location where 2 is negative
E) location where is zero

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
65
Orbitals with the same energy are called___________.
A) Quantum orbitals
B) Atomic orbitals
C) Antibonding orbitals
D) Bonding orbitals
E) Degenerate orbitals
A) Quantum orbitals
B) Atomic orbitals
C) Antibonding orbitals
D) Bonding orbitals
E) Degenerate orbitals

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following principle states that "Each orbital can accommodate a maximum of two electrons with opposite spin"?
A) Aufbau principle
B) Pauli exclusion principle
C) Hund's Rule
D) Heizenberg Uncertainty principle
E) Le Chatelier principle
A) Aufbau principle
B) Pauli exclusion principle
C) Hund's Rule
D) Heizenberg Uncertainty principle
E) Le Chatelier principle

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following represents ground state electron configuration for phosphorous?
A) 1s2 2s2 2p6 3s1 3p4
B) 1s2 2s2 2p6 3s2 3p4
C) 1s2 2s2 2p6 3s2 3p3
D) 1s2 2s2 2p6 3s2 3p2
E) 1s2 2s2 2p6 3s2 3p5
A) 1s2 2s2 2p6 3s1 3p4
B) 1s2 2s2 2p6 3s2 3p4
C) 1s2 2s2 2p6 3s2 3p3
D) 1s2 2s2 2p6 3s2 3p2
E) 1s2 2s2 2p6 3s2 3p5

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
68
For the following compound, identify each bond as polar covalent, nonpolar covalent or ionic and place a + on the most electropositive carbon. 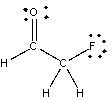


Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
69
Which element has the following electronic configuration? 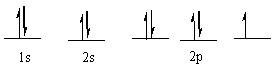
A) boron
B) carbon
C) silicon
D) nitrogen
E) fluorine
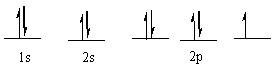
A) boron
B) carbon
C) silicon
D) nitrogen
E) fluorine

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following principle states "When orbitals of equal energy are available, every orbital gets one electron before any gets two electrons"?
A) Aufbau principle
B) Pauli exclusion principle
C) Hund's Rule
D) Heizenberg Uncertainty principle
E) Le Chatelier principle
A) Aufbau principle
B) Pauli exclusion principle
C) Hund's Rule
D) Heizenberg Uncertainty principle
E) Le Chatelier principle

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
71
The following ground state electron configuration violates ____. 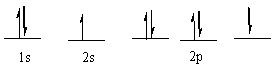
A) the Aufbau principle
B) the Pauli Exclusion principle
C) Hund's Rule
D) Heisenberg's Uncertainty principle
E) None of these
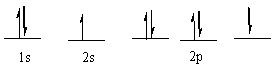
A) the Aufbau principle
B) the Pauli Exclusion principle
C) Hund's Rule
D) Heisenberg's Uncertainty principle
E) None of these

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
72
Which element has the electron configuration 1s2 2s2 2p6 3s2 3p3?
A) Cl
B) S
C) P
D) Al
E) N
A) Cl
B) S
C) P
D) Al
E) N

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
73
The following ground state electron configuration violates ___. 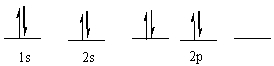
A) the Aufbau principle
B) the Pauli Exclusion principle
C) Hund's Rule
D) Heisenberg's Uncertainty principle
E) None of these
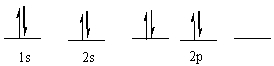
A) the Aufbau principle
B) the Pauli Exclusion principle
C) Hund's Rule
D) Heisenberg's Uncertainty principle
E) None of these

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
74
The atomic number for nitrogen is 7. Which of the following represents ground state electron configuration for nitrogen?
A) 1s2 2s1 2p4
B) 1s2 2p5
C) 2s2 2p5
D) 1s2 2s2 2p3
E) 1s2 2s2 3s3
A) 1s2 2s1 2p4
B) 1s2 2p5
C) 2s2 2p5
D) 1s2 2s2 2p3
E) 1s2 2s2 3s3

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
75
What is the letter designation for the following atomic orbital? 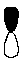
A) s
B) p
C) d
D) f
E) g
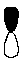
A) s
B) p
C) d
D) f
E) g

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
76
What is the electronic configuration for the nitride ion?
A) 1s2 2s2 2p0
B) 1s2 2s2 2p2
C) 1s2 2s22p3
D) 1s2 2s22p4
E) 1s2 2s2 2p6
A) 1s2 2s2 2p0
B) 1s2 2s2 2p2
C) 1s2 2s22p3
D) 1s2 2s22p4
E) 1s2 2s2 2p6

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
77
The following ground state electron configuration violates ____. 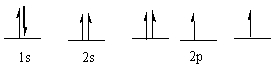
A) the Aufbau principle
B) the Pauli Exclusion principle
C) Hund's Rule
D) Heisenberg's Uncertainty principle
E) None of these
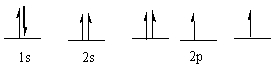
A) the Aufbau principle
B) the Pauli Exclusion principle
C) Hund's Rule
D) Heisenberg's Uncertainty principle
E) None of these

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
78
What is the electronic configuration for the oxide ion?
A) 1s2 2s2 2p6
B) 1s2 2s2 2p2
C) 1s2 2s22p4
D) 1s2 2s02p6
E) 1s2 2s2 2p63s22p2
A) 1s2 2s2 2p6
B) 1s2 2s2 2p2
C) 1s2 2s22p4
D) 1s2 2s02p6
E) 1s2 2s2 2p63s22p2

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
79
What is the electronic configuration for the magnesium ion?
A) 1s2 2s2 2p63s2
B) 1s2 2s2 2p6
C) 1s2 2s22p4
D) 1s2 2s22p63s1
E) 1s2 2s2 2p63s22p2
A) 1s2 2s2 2p63s2
B) 1s2 2s2 2p6
C) 1s2 2s22p4
D) 1s2 2s22p63s1
E) 1s2 2s2 2p63s22p2

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
80
Which element has the following electronic configuration? 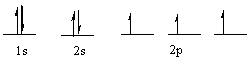
A) boron
B) carbon
C) silicon
D) nitrogen
E) fluorine
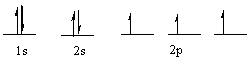
A) boron
B) carbon
C) silicon
D) nitrogen
E) fluorine

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck



