Deck 7: Structure and Synthesis of Alkenes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/128
Play
Full screen (f)
Deck 7: Structure and Synthesis of Alkenes
1
Draw the line energy orbital diagram for the outer shell of an sp2 hybridized carbon atom and explain how a carbon/carbon double is formed.
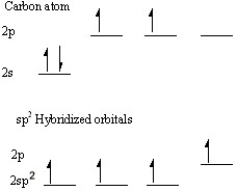 If we follow Hund's rule and fill each orbital with one electron and then promote one to the lone atomic p orbital, there are single electrons in the three sp hybrid orbitals that can form sigma overlaps with 2-H orbitals and one other sp2 hybridized carbon orbital. The atomic p orbital with one electron can then form a pi bond by a side to side overlap with the other p orbital from the other sp2 hybridized carbon atom.
If we follow Hund's rule and fill each orbital with one electron and then promote one to the lone atomic p orbital, there are single electrons in the three sp hybrid orbitals that can form sigma overlaps with 2-H orbitals and one other sp2 hybridized carbon orbital. The atomic p orbital with one electron can then form a pi bond by a side to side overlap with the other p orbital from the other sp2 hybridized carbon atom. 2
How many elements of unsaturation are implied by the molecular formula C7H11Cl?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4
2
3
Which of the following molecular formulas corresponds to a monocyclic saturated compound?
A) C6H6
B) C3H7Br
C) C3H7N
D) C3H8O
A) C6H6
B) C3H7Br
C) C3H7N
D) C3H8O
C3H7N
4
How many elements of unsaturation are implied by the molecular formula C5H8O?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following best approximates the CCC bond angle of propene?
A) 90°
B) 109°
C) 120°
D) 150°
E) 180°
A) 90°
B) 109°
C) 120°
D) 150°
E) 180°

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
6
A newly isolated natural product was found to have the molecular formula C15H28O2. By hydrogenating a sample of the compound, it was determined to possess one p bond. How many rings are present in the compound?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
7
Consider molecules with the formula C10H16. Which of the following structural features are not possible within this set of molecules?
A) 2 triple bonds
B) 1 ring and 1 triple bond
C) 2 rings and 1 double bond
D) 2 double bonds and 1 ring
E) 3 double bonds
A) 2 triple bonds
B) 1 ring and 1 triple bond
C) 2 rings and 1 double bond
D) 2 double bonds and 1 ring
E) 3 double bonds

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
8
The steroid testosterone has the molecular formula C19H28O2. Given that there are two P -bond in a molecule of testosterone, how many rings are present in each molecule?
A) 2
B) 3
C) 4
D) 5
E) 6
A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
9
How many elements of unsaturation do molecules with a molecular formula of C6H6Cl6 have?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
10
The prostaglandin precursor arachidonic acid has the molecular formula C20H32O2. Given that arachidonic acid is an acyclic carboxylic acid that contains no carbon-carbon triple bonds, how many carbon-carbon double bonds are present?
A) 2
B) 3
C) 4
D) 5
E) 6
A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
11
What two atomic orbitals or hybrid atomic orbitals overlap to form the CC π bond in ethylene?
A) C sp3 + C sp3
B) C sp3 + C sp2
C) C sp2 + C sp2
D) C sp2 + C p
E) C p + C p
A) C sp3 + C sp3
B) C sp3 + C sp2
C) C sp2 + C sp2
D) C sp2 + C p
E) C p + C p

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following statements best describes the relative bond dissociation energies of the sigma and pi bonds present in the carbon-carbon double bond of an alkene?
A) sigma > pi
B) pi > sigma
C) sigma = pi
D) cannot be estimated
A) sigma > pi
B) pi > sigma
C) sigma = pi
D) cannot be estimated

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
13
What two atomic orbitals or hybrid atomic orbitals overlap to form the CC σ bond in ethylene?
A) C sp3 + C sp3
B) C sp3 + C sp2
C) C sp2 + C sp2
D) C sp3 + C p
E) C sp2 + C p
A) C sp3 + C sp3
B) C sp3 + C sp2
C) C sp2 + C sp2
D) C sp3 + C p
E) C sp2 + C p

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
14
What two atomic orbitals or hybrid atomic orbitals overlap to form the CH bond in ethylene?
A) C sp3 + H s
B) C sp2 + H s
C) C sp + H s
D) C p + H s
A) C sp3 + H s
B) C sp2 + H s
C) C sp + H s
D) C p + H s

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
15
Circle all atoms that are coplanar in the molecule below. 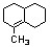


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
16
How many elements of unsaturation do molecules with a molecular formula of C8H4N2 have?
A) 2
B) 4
C) 6
D) 8
E) 10
A) 2
B) 4
C) 6
D) 8
E) 10

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
17
How many elements of unsaturation are implied by the molecular formula C6H12?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
18
The carbon-carbon bond length in ethylene is ________ than the carbon-carbon bond length in ethane, and the HCH bond angle in ethylene is ________ the HCH bond angle in ethane
A) shorter; smaller than
B) shorter; larger than
C) longer; smaller than
D) longer; larger than
E) longer; the same as
A) shorter; smaller than
B) shorter; larger than
C) longer; smaller than
D) longer; larger than
E) longer; the same as

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
19
Why is rotation about the carbon-carbon double bond in alkenes prohibited while relatively free rotation can occur about the carbon-carbon single bond in alkanes?

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
20
Carbon-carbon single bonds tend to be ________ and ________ than carbon-carbon double bonds.
A) shorter, stronger
B) longer, stronger
C) shorter, weaker
D) longer, weaker
A) shorter, stronger
B) longer, stronger
C) shorter, weaker
D) longer, weaker

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
21
Draw an acceptable structure for 4-ethylhept-1-ene.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
22
Draw and name all alkenes which have the molecular formula C4H8.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
23
Provide a correct IUPAC name for the structure below. 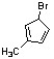


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
24
A chemist has isolated a new natural product and determined its molecular formula to be C24H40O4. In hydrogenation experiments the chemist found that each mole of the natural product reacted with two moles of H2. How many rings are present in the structure of the new natural product?
A) 2
B) 3
C) 4
D) 5
E) 6
A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
25
Provide the proper IUPAC name for the alkene shown below. 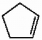


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following alkenes can show geometric isomerism: 2,3-dichloro-2-pentene, 4-chloro-3-ethyl-3-hexene, 3-chloro-2-methyl-2-butene, and 3-chloro-2-methyl-1-butene?

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
27
Draw an acceptable structure for 1,2-dimethylcyclohexene.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
28
Draw an acceptable structure for 4-phenylbut-1-ene.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
29
Identify the correct name for the following structure. 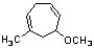
A) 3-methoxy-5-methylcyclohepta-1,5-diene
B) 6-methoxy-1-methylcyclohepta-1,4-diene
C) 7-methoxy-5-methylcyclohepta-1,4-diene
D) 4-methoxy-2-methylcyclohepta-1,5-diene

A) 3-methoxy-5-methylcyclohepta-1,5-diene
B) 6-methoxy-1-methylcyclohepta-1,4-diene
C) 7-methoxy-5-methylcyclohepta-1,4-diene
D) 4-methoxy-2-methylcyclohepta-1,5-diene

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
30
Provide an acceptable name for (CH3CH2)2CHCH2CH=CH2.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
31
Provide the proper IUPAC name for the alkene shown below.
CH2=CHCH2CH2CH2CH3
CH2=CHCH2CH2CH2CH3

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
32
How many elements of unsaturation are implied by the molecular formula C5H5NO2?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
33
Name the alkene shown. Be sure to include the appropriate E or Z label necessary. 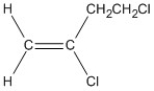


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
34
Provide an acceptable name for (CH3)2CHCH=C(CH3)CH2CH3.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
35
Provide the proper IUPAC name for the alkene shown below. 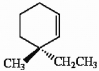


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
36
Draw and name the six alkenes which have the molecular formula C5H10.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
37
Provide the proper IUPAC name for the alkene shown below. 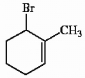


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
38
Name the compound shown below. 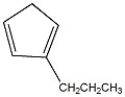


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
39
How many elements of unsaturation are implied by the molecular formula C8H11N?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
40
For which of the following alkenes will cis- and trans- isomers not exist? 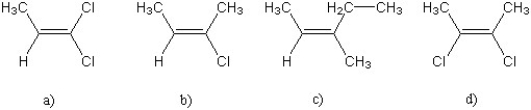
A) a) only
B) b) only
C) both a) and c)
D) d) only
E) both c) and d)

A) a) only
B) b) only
C) both a) and c)
D) d) only
E) both c) and d)

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following best describes the geometry about the carbon-carbon double bond in the alkene below? 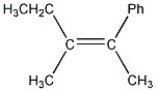
A) E
B) Z
C) neither E nor Z

A) E
B) Z
C) neither E nor Z

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following best describes the geometry about the carbon-carbon double bond in the alkene below? 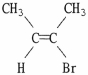
A) E
B) Z
C) neither E nor Z

A) E
B) Z
C) neither E nor Z

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following best describes the geometry about the carbon-carbon double bond in the alkene below? 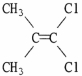
A) E
B) Z
C) neither E nor Z

A) E
B) Z
C) neither E nor Z

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
44
Which has the smaller heat of hydrogenation, (E)-2-pentene or (Z)-2-pentene? What is the structural origin of this difference?

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
45
Draw the structure of polyethylene.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
46
Circle the alkene below which has the smallest heat of hydrogenation. 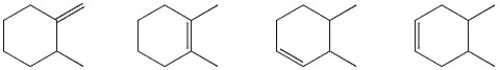


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
47
Provide the proper IUPAC name for the alkene shown below. 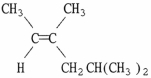


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
48
Provide the proper IUPAC name for the alkene shown below. 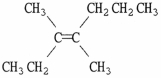


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
49
Provide the proper IUPAC name for the alkene shown below. 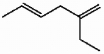


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
50
Draw an acceptable structure for (Z)-2-chloro-4-ethylhex-2-ene.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following best describes the geometry about the carbon-carbon double bond in the alkene below? 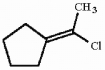
A) E
B) Z
C) neither E nor Z

A) E
B) Z
C) neither E nor Z

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
52
Translate the following condensed structure to a line-angle structure.
(E) CH3CBrCH(CH2)2C(O)CH(CH3)2
(E) CH3CBrCH(CH2)2C(O)CH(CH3)2

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
53
The compound produced when 3-methylpent-2-ene undergoes hydrogenation in the presence of a platinum catalyst is ________.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
54
Provide the structure of the monomer from which PVC or poly(vinyl chloride) is made.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
55
Provide a structure for (Z)-4-bromo-3-heptene.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
56
Name the alkene shown. Be sure to include the appropriate E or Z label necessary. 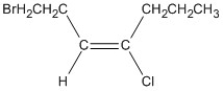


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
57
Provide the structure of (Z)-1-chloro-1-fluoro-1-butene.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
58
Draw the structure of poly(tetrafluoroethylene) or Teflon.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
59
Provide the proper IUPAC name for the alkene shown below. 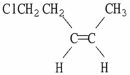


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following best describes the geometry about the carbon-carbon double bond in the alkene below? 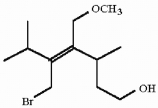
A) E
B) Z
C) neither E nor Z

A) E
B) Z
C) neither E nor Z

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
61
Draw the alkene of formula C5H10 which evolves the most heat per mole upon hydrogenation.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
62
Compare the relative heats of hydrogenation of cyclobutene and cyclopentene and explain the difference in magnitude.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
63
The trans isomers of cycloalkenes with rings containing fewer than ________ atoms are unstable at room temperature.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
64
Does the alkene shown below violate Bredt's rule? 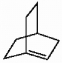


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
65
Which sequence ranks the following compounds in order of increasing heat of hydrogenation, ΔHhyd?
1. cis-2-butene
2. 1-butene
3. cyclohexene
A) 3<1<2
B) 3<2<1
C) 2<1<3
D) 1<3<2
1. cis-2-butene
2. 1-butene
3. cyclohexene
A) 3<1<2
B) 3<2<1
C) 2<1<3
D) 1<3<2

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
66
Circle the most stable alkene in the set of isomers below. 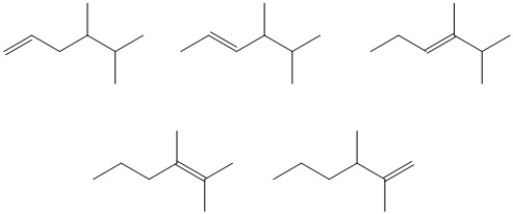


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
67
Using Zaitsev's rule, choose the most stable alkene among the following.
A) 1,2-dimethylcyclohexene
B) 1,6-dimethylcyclohexene
C) cis-3,4-dimethylcyclohexene
D) They are all of equal stability according to Zaitsev's rule.
A) 1,2-dimethylcyclohexene
B) 1,6-dimethylcyclohexene
C) cis-3,4-dimethylcyclohexene
D) They are all of equal stability according to Zaitsev's rule.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
68
Circle the alkene isomer that has the largest heat of hydrogenation. 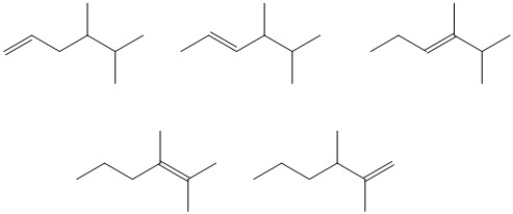


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
69
Circle the most stable alkene in the set of isomers below. 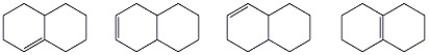


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following alkenes has the largest molar heat of hydrogenation (ie, releases the most heat upon hydrogenation)?
A) 2,3-dimethyl-2-butene
B) 2-methyl-2-butene
C) -2-butene
D) -2-butene
E) 1-hexene
A) 2,3-dimethyl-2-butene
B) 2-methyl-2-butene
C) -2-butene
D) -2-butene
E) 1-hexene

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following cycloalkenes would be expected to be stable? 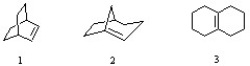
A) 1 & 2
B) 2 & 3
C) 2
D) 1 & 3
E) None are stable.

A) 1 & 2
B) 2 & 3
C) 2
D) 1 & 3
E) None are stable.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
72
Using Zaitsev's rule, choose the most stable alkene among the following.
A) hex-1-ene
B) (E)-hex-2-ene
C) (Z)-hex-2-ene
D) They are all of equal stability according to Zaitsev's rule.
A) hex-1-ene
B) (E)-hex-2-ene
C) (Z)-hex-2-ene
D) They are all of equal stability according to Zaitsev's rule.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
73
There are three isomeric methylbutene structures. Draw each of them and then circle the isomer with the highest heat of hydrogenation.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
74
Does the alkene shown below violate Bredt's rule? 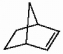


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
75
Draw all likely alkene products in the following reaction and circle the product you expect to predominate. 


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
76
Does the alkene shown below violate Bredt's rule? 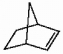


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
77
Using Zaitsev's rule, choose the most stable alkene among the following.
A) 1-methylcyclohexene
B) 3-methylcyclohexene
C) 4-methylcyclohexene
D) They are all of equal stability according to Zaitsev's rule.
A) 1-methylcyclohexene
B) 3-methylcyclohexene
C) 4-methylcyclohexene
D) They are all of equal stability according to Zaitsev's rule.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following alkenes has the smallest molar heat of hydrogenation (ie, releases the least heat upon hydrogenation)?
A) 2,3-dimethyl-2-butene
B) 2-methyl-2-butene
C) -2-butene
D) -2-butene
E) 1-hexene
A) 2,3-dimethyl-2-butene
B) 2-methyl-2-butene
C) -2-butene
D) -2-butene
E) 1-hexene

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
79
Does the alkene shown below violate Bredt's rule? 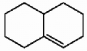


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
80
Consider the constitutional isomers 2-methylbut-1-ene, 2-methylbut-2-ene, and 3-methylbut-1-ene. When each of these alkenes is subjected to catalytic hydrogenation (H2, Pt), a single product results. Which of the following best describes the structural relationship among these products?
A) The products are cis-trans isomers.
B) The products are identical.
C) The products are constitutional isomers.
D) The products are enantiomers.
E) The products are diastereomers.
A) The products are cis-trans isomers.
B) The products are identical.
C) The products are constitutional isomers.
D) The products are enantiomers.
E) The products are diastereomers.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck



