Deck 14: Ethers, Epoxides, and Thioethers
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/127
Play
Full screen (f)
Deck 14: Ethers, Epoxides, and Thioethers
1
Give two properties of ethers which allow them to be commonly used as solvents in organic reactions.
(any two of the following)
1. Dissolve a wide variety of both polar and nonpolar compounds.
2. Tend to be unreactive under a large number of reaction conditions.
3. Have low boiling points; easily removed from product mixture.
1. Dissolve a wide variety of both polar and nonpolar compounds.
2. Tend to be unreactive under a large number of reaction conditions.
3. Have low boiling points; easily removed from product mixture.
2
Provide the IUPAC name of the compound shown below. 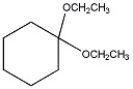

1,1-diethoxycyclohexane
3
Which of the following corresponds to the COC bond angle in dimethyl ether?
A) 60°
B) 94°
C) 110°
D) 122°
E) 180°
A) 60°
B) 94°
C) 110°
D) 122°
E) 180°
110°
4
Provide the structure of 3-methoxyfuran.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
5
Provide a structural representation of 1,2-epoxybutane (also called 2-ethyloxirane).

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
6
Provide a structural representation of isopropyl tert-butyl ether.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
7
What is the hybridization of the oxygen atom in dialkyl ethers?
A) sp3
B) sp2
C) sp
D) s
E) p
A) sp3
B) sp2
C) sp
D) s
E) p

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
8
What is the correct IUPAC name for the following compound? 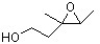
A) 3-methyl-3,4-epoxypentan-1-ol
B) 5-hydroxy-3-methyl-2,3-epoxypentane
C) 1-(2-hydroxyethyl)-1,2-dimethyloxirane
D) 3,4-epoxy-3-methylpentan-1-ol

A) 3-methyl-3,4-epoxypentan-1-ol
B) 5-hydroxy-3-methyl-2,3-epoxypentane
C) 1-(2-hydroxyethyl)-1,2-dimethyloxirane
D) 3,4-epoxy-3-methylpentan-1-ol

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
9
Provide the structure of 4,4-dimethylpyran.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
10
What is the complete systematic IUPAC name for the following compound? 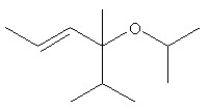
A) 4-(1-methylethoxy)-4-isopropyl-4-methylpent-2-ene
B) 4-isopropyl-2,4-dimethylhept-5-en-3-ol
C) isopropyl-(4-isopropyl-4-methylbut-2-enyl) ether
D) (E)-4-isopropoxy-4,5-dimethylhex-2-ene
E) 4-isopropyl-4-methylbut-2-en-isopropyl ether

A) 4-(1-methylethoxy)-4-isopropyl-4-methylpent-2-ene
B) 4-isopropyl-2,4-dimethylhept-5-en-3-ol
C) isopropyl-(4-isopropyl-4-methylbut-2-enyl) ether
D) (E)-4-isopropoxy-4,5-dimethylhex-2-ene
E) 4-isopropyl-4-methylbut-2-en-isopropyl ether

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is not a property of ethers which makes them good solvents in organic reactions?
A) They dissolve a wide range of polar substances.
B) They have relatively high boiling points for their molecular weights.
C) They are nonhydroxylic.
D) They dissolve a wide range of nonpolar substances.
E) They are normally unreactive toward strong bases.
A) They dissolve a wide range of polar substances.
B) They have relatively high boiling points for their molecular weights.
C) They are nonhydroxylic.
D) They dissolve a wide range of nonpolar substances.
E) They are normally unreactive toward strong bases.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
12
Anisole is known by two other names. Give either of them.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
13
Provide a structural representation of cis-3-ethyl-1,2-epoxycyclopentane.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
14
What complex results when BF3 is dissolved in dimethyl ether?

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
15
Which has the higher boiling point, diethyl ether or butan-1-ol? Briefly explain.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
16
Draw structures which show the hydrogen bonding interaction that exists between water and dimethyl ether.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
17
Provide a structural representation of 3-ethylfuran.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
18
Provide a structural representation of 2-ethoxypentane.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
19
Provide a structural representation of oxetane.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following is not a true statement?
A) Dioxanes are six-membered ring ethers.
B) Furans are five-membered ring ethers.
C) Oxiranes are three-membered ring ethers.
D) Oxetanes are five-membered ring ethers.
E) Pyrans are six-membered-ring ethers.
A) Dioxanes are six-membered ring ethers.
B) Furans are five-membered ring ethers.
C) Oxiranes are three-membered ring ethers.
D) Oxetanes are five-membered ring ethers.
E) Pyrans are six-membered-ring ethers.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
21
Provide the major organic product in the reaction below.
CH3CH2CH2OH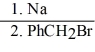
CH3CH2CH2OH
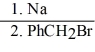

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
22
Provide the major organic product of the following. 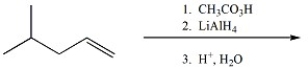
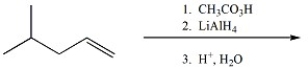

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following is an acceptable way to synthesize t-butyl ethyl ether?
A) treatment of t-butyl bromide with sodium ethoxide
B) treatment of ethyl bromide with sodium t-butoxide
C) heating a mixture of ethanol and t-butanol in sulfuric acid
D) treating t-butyl bromide with Hg(OAc)2
E) treating t-butanolwith Hg(OAc)2
A) treatment of t-butyl bromide with sodium ethoxide
B) treatment of ethyl bromide with sodium t-butoxide
C) heating a mixture of ethanol and t-butanol in sulfuric acid
D) treating t-butyl bromide with Hg(OAc)2
E) treating t-butanolwith Hg(OAc)2

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
24
Propose a structure for the ether of formula C4H10O with the following 1H NMR signals:
δ 1.13 (doublet, 6H), 3.30 (singlet, 3H), 3.65 (septet, 1H) (ppm).
δ 1.13 (doublet, 6H), 3.30 (singlet, 3H), 3.65 (septet, 1H) (ppm).

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
25
Propose a structure for the ether of formula C4H10O with the following 1H NMR signals:
δ 0.95 (triplet, 3H), 1.52 (multiplet, 2H), 3.30 (singlet, 3H), 3.40 (triplet, 2H) (ppm).
δ 0.95 (triplet, 3H), 1.52 (multiplet, 2H), 3.30 (singlet, 3H), 3.40 (triplet, 2H) (ppm).

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
26
Provide the major organic product of the following. 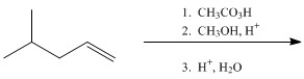
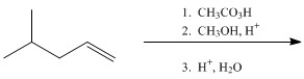

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
27
Provide an acceptable name for the compound shown below. 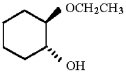


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
28
Provide an acceptable name for the compound shown below.
CH3OCH2CH(CH3)2
CH3OCH2CH(CH3)2

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
29
Provide the major organic product of the following. 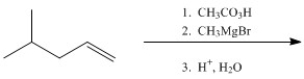
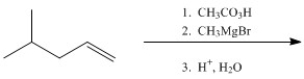

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
30
Provide the structure of the fragment in the mass spectrum of dipropyl ether that results from a cleavage.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
31
The Williamson ether synthesis occurs by the ________ mechanistic pathway.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
32
In mass spectrometry, what is the most common fragmentation of ethers?

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
33
Provide an acceptable name for the compound shown below. 


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
34
Provide the major organic product in the reaction below. 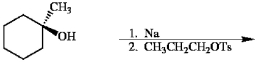
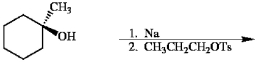

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
35
What is the chemical shift of the carbon bound to oxygen in ethers in 13C NMR?

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
36
Propose a structure for the ether of formula C4H10O with the following 1H NMR signals:
δ 1.20 (triplet, 6H), δ 3.45 (quartet, 4H) (ppm).
δ 1.20 (triplet, 6H), δ 3.45 (quartet, 4H) (ppm).

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
37
Which pair of reagents would produce the highest yield of (R)-2-ethoxybutane?
A) sodium (S)-2-butoxide + iodoethane
B) sodium (R)-2-butoxide + iodoethane
C) sodium ethoxide + (S)-2-iodobutane
D) sodium ethoxide + (R)-2-iodobutane
E) Both B and C would work equally well.
A) sodium (S)-2-butoxide + iodoethane
B) sodium (R)-2-butoxide + iodoethane
C) sodium ethoxide + (S)-2-iodobutane
D) sodium ethoxide + (R)-2-iodobutane
E) Both B and C would work equally well.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
38
Show the best method for preparing methoxycyclopentane via the Williamson ether synthesis.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
39
Provide the major organic product of the following. 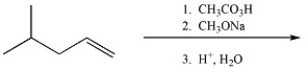
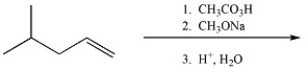

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
40
Provide an acceptable name for the compound shown below. 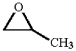


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
41
Provide the major organic product of the reaction shown below. 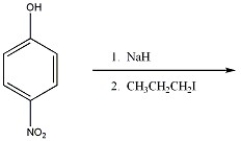


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
42
Provide the major organic product of the following. 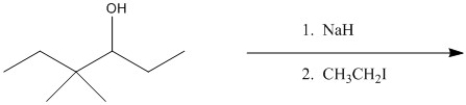
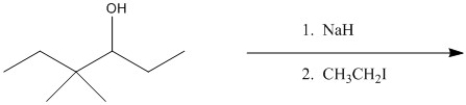

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
43
Provide the major organic product of the following. 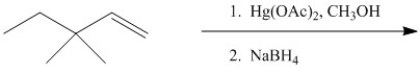
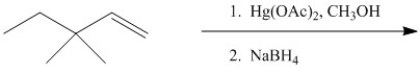

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
44
Provide the sequence of reactions by which propoxycyclohexane can be prepared through a Williamson ether synthesis.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
45
Show the reagents necessary to prepare 1-ethoxy-1-methylcyclopentane from 1-methylcyclopentene.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
46
Show the reagents necessary for the conversion of 1-bromo-1-methylcyclopentane to
1-ethoxy-1-methylcyclopentane.
1-ethoxy-1-methylcyclopentane.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
47
Show the best Williamson ether synthesis for the following target molecule. 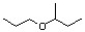


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
48
Provide the major organic product of the following reactions. 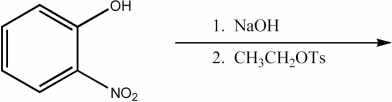
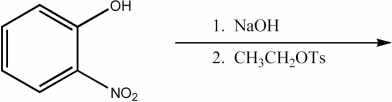

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
49
Complete the following reaction by drawing a structure for the expected product. 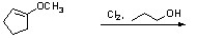
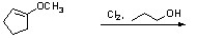

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
50
Provide the major organic product of the following reactions. 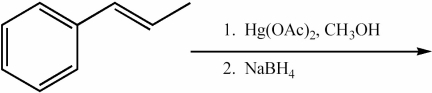


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
51
Provide the major organic product of the reaction shown below. 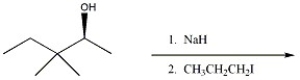
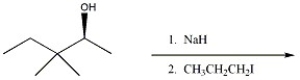

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
52
Provide the necessary reagents to complete the following reaction. 


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following reactions is classified as a Williamson ether synthesis?
A)
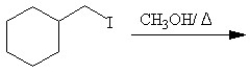
B)
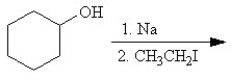
C)
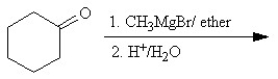
D)
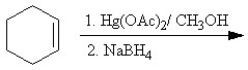
E)
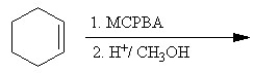
A)

B)

C)
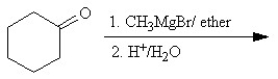
D)
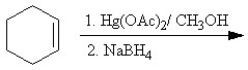
E)


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following reactions or series of reactions will lead to the formation of methoxycyclohexane?
A)
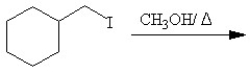
B)
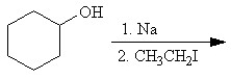
C)
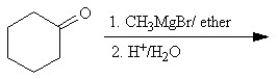
D)
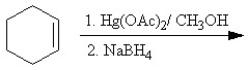
E)
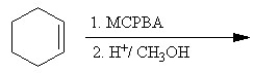
A)

B)

C)
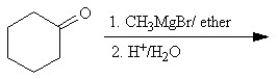
D)
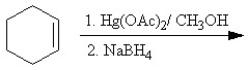
E)


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
55
Provide the major organic product in the reaction below.
CH3CH2CH=CH2
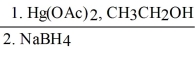 →
→
CH3CH2CH=CH2
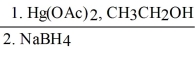 →
→
Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
56
When cyclohexene is subjected to mercuration in methanol and the resulting mixture is reduced with sodium borohydride, the major organic product is:
A) a meso ether.
B) a 1:1 mixture of enantiomeric ethers.
C) a meso diol.
D) a 1:1 mixture of enantiomeric diols.
E) methoxycyclohexane.
A) a meso ether.
B) a 1:1 mixture of enantiomeric ethers.
C) a meso diol.
D) a 1:1 mixture of enantiomeric diols.
E) methoxycyclohexane.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
57
Show the best method for preparing 4-propoxytoluene via the Williamson ether synthesis.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
58
Provide the major organic product of the following reactions. 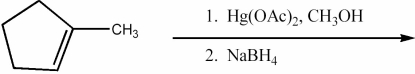
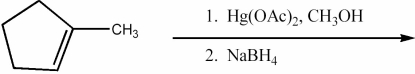

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
59
Show how propyl cyclopentyl ether can be prepared starting with cyclopentene and using an alkoxymercuration-demercuration route.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
60
When pent-1-ene is treated with mercury(II) acetate in methanol and the resulting product is reacted with NaBH4, what is the primary organic compound which results?
A) 3-ethoxypentane
B) 1-methoxypentane
C) 1-ethoxypentane
D) 2-ethoxypentane
E) 2-methoxypentane
A) 3-ethoxypentane
B) 1-methoxypentane
C) 1-ethoxypentane
D) 2-ethoxypentane
E) 2-methoxypentane

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
61
When hexan-1-ol is treated with conc. H2SO4 at moderate temperatures, ________ is formed via a(n) ________ mechanism.
A) di-n-hexyl ether, SN2
B) di-n-hexyl ether, SN1
C) di-n-hexyl ether, E2
D) di-n-propyl ether, E1
E) hex-1-ene, SN1
A) di-n-hexyl ether, SN2
B) di-n-hexyl ether, SN1
C) di-n-hexyl ether, E2
D) di-n-propyl ether, E1
E) hex-1-ene, SN1

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
62
Can one prepare di-sec-butyl ether in good yield by heating butan-2-ol in the presence of sulfuric acid? Explain.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
63
Treatment of tetrahydrofuran with excess HBr results in the formation of what major organic product?
A) 1-bromobutane
B) 1,2-dibromobutane
C) 1,4-dibromobutane
D) 1-bromopentane
E) 1,5-dibromopentane
A) 1-bromobutane
B) 1,2-dibromobutane
C) 1,4-dibromobutane
D) 1-bromopentane
E) 1,5-dibromopentane

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following compounds would undergo autoxidation most readily?
A) dibenzyl ether
B) MTBE
C) diethyl ether
D) isobutyl phenyl ether
A) dibenzyl ether
B) MTBE
C) diethyl ether
D) isobutyl phenyl ether

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
65
Di-n-pentyl ether can be converted to 1-bromopentane by treatment with HBr through essentially a(n) ________ mechanism.
A) SN2
B) SN1
C) E2
D) E1
E) ring opening
A) SN2
B) SN1
C) E2
D) E1
E) ring opening

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
66
Provide the major organic product of the reaction shown below. 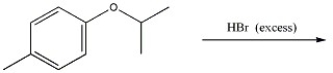


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
67
What are the expected products of the reaction of PhOCH3 with concentrated HI?
A) phenol and methanol
B) phenol and iodomethane
C) iodobenzene and methanol
D) iodobenzene and iodomethane
A) phenol and methanol
B) phenol and iodomethane
C) iodobenzene and methanol
D) iodobenzene and iodomethane

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
68
Provide the major organic product in the reaction below. 


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
69
When ethers are stored in the presence of oxygen, what explosive materials can result from autoxidation of the ether?

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
70
Provide the major organic product of the following reactions. 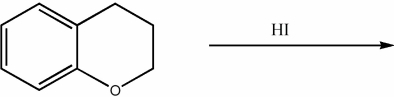
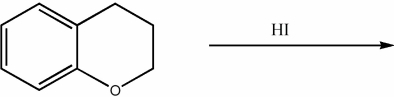

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
71
Provide the major organic product of the reaction shown below. 


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
72
Provide two reasons why it would be difficult to prepare ethoxycyclopentane via an intermolecular dehydration route from ethanol and cyclopentanol.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
73
Provide the major organic product(s) in the reaction below. 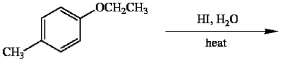
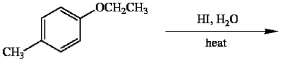

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
74
Predict the products of the following reaction and give a reasonable mechanism for their formation. 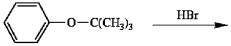
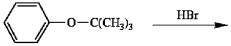

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
75
What is the major organic product which results when tetrahydrofuran is reacted with excess HBr?
A) 1,2-dibromobutane
B) 1,3-dibromobutane
C) 1,4-dibromobutane
D) 4-bromobutan-1-ol
E) 3-bromobutan-1-ol
A) 1,2-dibromobutane
B) 1,3-dibromobutane
C) 1,4-dibromobutane
D) 4-bromobutan-1-ol
E) 3-bromobutan-1-ol

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
76
Some ethers can be prepared by heating the appropriate alcohol in the presence of sulfuric acid. Can di-sec-butyl ether be prepared from sec-butanol by this route? Explain your answer.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
77
Provide the major organic product of the following. 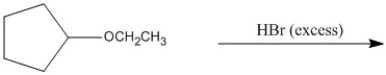
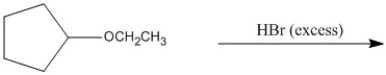

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
78
Suggest a reasonable mechanism for the reaction shown below. 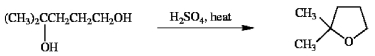
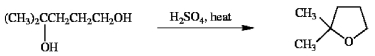

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
79
Provide the major organic product in the reaction below. 


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
80
The presence of what product of the autooxidation of ethers makes the distillation of ethers dangerous?

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck



