Deck 6: Alkyl Halides; Nucleophilic Substitution
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/137
Play
Full screen (f)
Deck 6: Alkyl Halides; Nucleophilic Substitution
1
Which of the following is a geminal dihalide?
A) cis-1,2-dibromocyclopentane
B) 3,3-dichloropentane
C) trans-1,4-diiodocyclohexane
D) isobutyl chloride
E) 1,3-dibromoheptane
A) cis-1,2-dibromocyclopentane
B) 3,3-dichloropentane
C) trans-1,4-diiodocyclohexane
D) isobutyl chloride
E) 1,3-dibromoheptane
3,3-dichloropentane
2
Which of the following best describes the carbon-chlorine bond of an alkyl chloride?
A) nonpolar; no dipole
B) polar; δ+ at carbon and δ- at chlorine
C) polar; δ- at carbon and δ+ at chlorine
D) ionic
E) none of the above
A) nonpolar; no dipole
B) polar; δ+ at carbon and δ- at chlorine
C) polar; δ- at carbon and δ+ at chlorine
D) ionic
E) none of the above
polar; δ+ at carbon and δ- at chlorine
3
Provide an acceptable name for CH3CH2CH2C(CH3)2I.
2-iodo-2-methylpentane
4
What general classification is given to the molecule below? 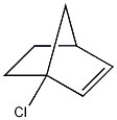
A) primary chloride
B) secondary chloride
C) tertiary chloride
D) vinyl chloride
E) benzyl chloride

A) primary chloride
B) secondary chloride
C) tertiary chloride
D) vinyl chloride
E) benzyl chloride

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
5
Provide the structure of isopropyl iodide.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is classified as a vinylic halide?
A) CH3CH CHOH
CHOH
B) CH3CH CHCl
CHCl
C) CH3CH CHCH2Cl
CHCH2Cl
D) CH3CH2CH2CH2Br
E) BrCH2CH CH2
CH2
A) CH3CH
 CHOH
CHOHB) CH3CH
 CHCl
CHClC) CH3CH
 CHCH2Cl
CHCH2ClD) CH3CH2CH2CH2Br
E) BrCH2CH
 CH2
CH2
Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
7
The flame retardant below has been found to be an androgen agonist and may have a contribution to an increasing rate of occurrence of prostate cancer (J. Med. Chem. 2006, 7366). What is the correct term that describes the relative position of the bromides in this structure? 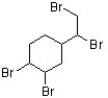
A) geminal
B) vinyl
C) vicinal
D) aryl

A) geminal
B) vinyl
C) vicinal
D) aryl

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is a secondary halide?
A) ClCH2CH2C(CH3)3
B) ClCH2CH2CH(CH3)2
C) (CH3)2CClCH2CH3
D) (CH3)3CCHClCH3
E) ClCH2C(CH3)CH2CH3
A) ClCH2CH2C(CH3)3
B) ClCH2CH2CH(CH3)2
C) (CH3)2CClCH2CH3
D) (CH3)3CCHClCH3
E) ClCH2C(CH3)CH2CH3

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
9
What is the IUPAC name of the compound shown below? 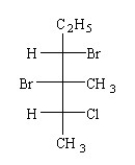
A) (2R,3S,4S)-2-chloro-3,4-dibromo-3-methylhexane
B) (2R,3S,4S)-3,4-dibromo-2-chloro-3-methylhexane
C) (3S,4S,5S)-3,4-dibromo-5-chloro-4-methylhexane
D) (2R,3R,4S)-3,4-dibromo-2-chloro-3-methylhexane
E) (2R,3S,4R)-3,4-dibromo-2-chloro-3-methylhexane

A) (2R,3S,4S)-2-chloro-3,4-dibromo-3-methylhexane
B) (2R,3S,4S)-3,4-dibromo-2-chloro-3-methylhexane
C) (3S,4S,5S)-3,4-dibromo-5-chloro-4-methylhexane
D) (2R,3R,4S)-3,4-dibromo-2-chloro-3-methylhexane
E) (2R,3S,4R)-3,4-dibromo-2-chloro-3-methylhexane

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
10
Provide the structure of 1-bromo-3-methylhexane.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
11
Provide an acceptable name for CHCl3.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
12
Draw the structure of cis-1,3-dichlorocyclopentane.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following is a primary alkyl halide?
A) methyl bromide
B) isopropyl bromide
C) t-butyl iodide
D) cyclohexyl bromide
E) isobutyl chloride
A) methyl bromide
B) isopropyl bromide
C) t-butyl iodide
D) cyclohexyl bromide
E) isobutyl chloride

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
14
Provide an acceptable name for the compound shown below. 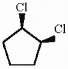


Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
15
Provide the name of the bromoalkane shown below. 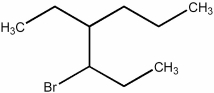


Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is a vicinal dihalide?
A) cis-1,2-dibromocyclopentane
B) 3,3-dichloropentane
C) trans-1,4-diiodocyclohexane
D) isobutyl chloride
E) 1,3-dibromoheptane
A) cis-1,2-dibromocyclopentane
B) 3,3-dichloropentane
C) trans-1,4-diiodocyclohexane
D) isobutyl chloride
E) 1,3-dibromoheptane

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following is a tertiary halide?
A) ClCH2CH2C(CH3)3
B) ClCH2CH2CH(CH3)2
C) (CH3)2CClCH2CH3
D) (CH3)3CCHClCH3
E) ClCH2C(CH3)CH2CH3
A) ClCH2CH2C(CH3)3
B) ClCH2CH2CH(CH3)2
C) (CH3)2CClCH2CH3
D) (CH3)3CCHClCH3
E) ClCH2C(CH3)CH2CH3

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
18
Provide an acceptable name for the compound shown below. 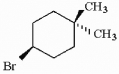


Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
19
What is the IUPAC name of the compound shown below? 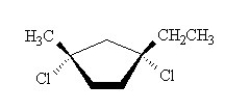
A) (1S,3S)-1,3-dichloro-1-ethyl-3-methylcyclopentane
B) (1R,3S)-1,3-dichloro-1-methyl-3-ethylcyclopentane
C) (1R,4S)-1,4-dichloro-1-ethyl-4-methylcyclopentane
D) (1R,3S)-1,3-dichloro-1-ethyl-3-methylcyclopentane
E) (1S,3R)-1,3-dichloro-3-ethyl-1-methylcyclopentane

A) (1S,3S)-1,3-dichloro-1-ethyl-3-methylcyclopentane
B) (1R,3S)-1,3-dichloro-1-methyl-3-ethylcyclopentane
C) (1R,4S)-1,4-dichloro-1-ethyl-4-methylcyclopentane
D) (1R,3S)-1,3-dichloro-1-ethyl-3-methylcyclopentane
E) (1S,3R)-1,3-dichloro-3-ethyl-1-methylcyclopentane

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following is a secondary alkyl halide?
A) methyl bromide
B) isopropyl chloride
C) t-butyl iodide
D) propyl bromide
E) isobutyl chloride
A) methyl bromide
B) isopropyl chloride
C) t-butyl iodide
D) propyl bromide
E) isobutyl chloride

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
21
Provide the structure of the major organic product in the reaction below. 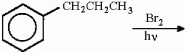
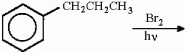

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
22
Provide a detailed, stepwise mechanism for the reaction below.
(CH3)2CHCH2CH2CH2I + CN- → (CH3)2CHCH2CH2CH2CH2CN + I-
(CH3)2CHCH2CH2CH2I + CN- → (CH3)2CHCH2CH2CH2CH2CN + I-

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
23
Provide the major organic product of the reaction below. 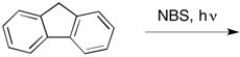


Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
24
Name the haloalkane shown. Be sure to include the appropriate R or S description if needed. 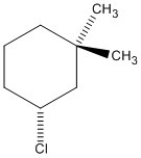


Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
25
Draw the structures of the two major products formed in the following reaction. Indicate which of the two structures would be predicted to predominate under thermodynamic condition and explain why. 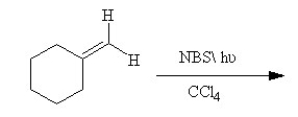


Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
26
Although F is more electronegative than Cl, the C-Cl bond has a larger dipole moment than the 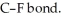
Explain.
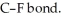
Explain.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
27
When water is shaken with dichloromethane, a two-phase system results. Which compound forms the upper phase? Explain.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following alkyl halides has the smallest molecular dipole moment?
A) CH3F
B) CH3Cl
C) CH2I2
D) CH2Cl2
E) CF4
A) CH3F
B) CH3Cl
C) CH2I2
D) CH2Cl2
E) CF4

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
29
Among the following alkyl halides, choose the one with the lowest boiling point.
A) t-butyl chloride
B) n-butyl chloride
C) t-butyl bromide
D) n-butyl iodide
E) t-butyl iodide
A) t-butyl chloride
B) n-butyl chloride
C) t-butyl bromide
D) n-butyl iodide
E) t-butyl iodide

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
30
What type of intermediate is present in the SN2 reaction of cyanide with bromoethane?
A) carbocation
B) free radical
C) carbene
D) carbanion
E) This reaction has no intermediate.
A) carbocation
B) free radical
C) carbene
D) carbanion
E) This reaction has no intermediate.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
31
Name the haloalkane shown. Be sure to include the appropriate R or S description if needed. 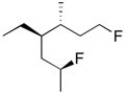


Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
32
The term CFC is an abbreviation for ________.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
33
When 2,2-dimethylbutane is subjected to free-radical chlorination, ________ distinct monochlorinated products are possible and ________ of these contain asymmetric carbon atoms.
A) 4; 2
B) 5; 0
C) 3; 0
D) 5; 2
E) 4; 0
A) 4; 2
B) 5; 0
C) 3; 0
D) 5; 2
E) 4; 0

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following best describes DDT?
A) a chlorocarbon used to decaffeinate coffee beans
B) a polybrominated organic compound used as a herbicide
C) a Freon used as a foaming agent
D) a mixed alkyl halide used as an anesthetic
E) a polychlorinated organic compound used as an insecticide
A) a chlorocarbon used to decaffeinate coffee beans
B) a polybrominated organic compound used as a herbicide
C) a Freon used as a foaming agent
D) a mixed alkyl halide used as an anesthetic
E) a polychlorinated organic compound used as an insecticide

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
35
The following structure is a proteasome inhibitor that may find application in treating cervical cancer (J. Med. Chem. 2011, 449). The halides in this structure may be classified as ________ and ________. 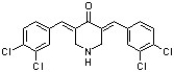
A) vicinal; vinyl
B) vicinal; aryl
C) vinyl; aryl
D) vinyl; geminal

A) vicinal; vinyl
B) vicinal; aryl
C) vinyl; aryl
D) vinyl; geminal

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
36
Show how the following reaction might be constructed using an SN2 reaction by drawing structures for the starting materials. 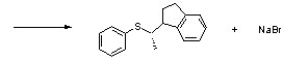


Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
37
Provide the structure of the major organic product in the reaction below. 


Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
38
Consider the reaction of (CH3)3CO- with iodomethane. Will the reaction rate increase, decrease, or remain the same if the concentration of iodomethane is increased? Explain.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
39
Draw two reasonable resonance structures and the hybrid of the intermediate formed in the following reaction. 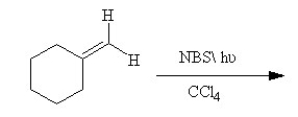


Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
40
Arrange the following alkyl halides in order of increasing boiling point:
CH3CH2Br, CH3CH2CH2Br, (CH3)2CHBr, CH3CH2F.
CH3CH2Br, CH3CH2CH2Br, (CH3)2CHBr, CH3CH2F.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following compounds will undergo an SN2 reaction most readily?
A) (CH3)3CCH2I
B) (CH3)3CCl
C) (CH3)2CHI
D) (CH3)2CHCH2CH2CH2I
E) (CH3)2CHCH2CH2CH2Cl
A) (CH3)3CCH2I
B) (CH3)3CCl
C) (CH3)2CHI
D) (CH3)2CHCH2CH2CH2I
E) (CH3)2CHCH2CH2CH2Cl

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
42
Which halide is a better nucleophile, iodide or fluoride? Explain.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
43
Arrange the following substrates in order of their increasing SN2 reactivity with NaCN:
bromoethane, 1-chloro-3,3-dimethylpentane, 1-chloro-2,2-dimethylpentane, and 2-bromo-2-methylpentane.
bromoethane, 1-chloro-3,3-dimethylpentane, 1-chloro-2,2-dimethylpentane, and 2-bromo-2-methylpentane.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
44
List the following bromides in order of their increasing reactivity as substrates in SN2 reactions: PhBr, PhCH2Br, and PhCH(CH3)Br.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
45
Do all primary iodides react with N3- at the same rate via the SN2 mechanism? Explain.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
46
Rank the species below in order of increasing nucleophilicity in hydroxylic solvents:
CH3CO2-, CH3S-, HO-, H2O.
CH3CO2-, CH3S-, HO-, H2O.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
47
Arrange the following species in order of increasing nucleophilicity:
CH3CH2S-, CH3CH2O-, (CH3)3CO-, (CH3)3COH.
CH3CH2S-, CH3CH2O-, (CH3)3CO-, (CH3)3COH.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following solvents could be described as polar and protic?
A) ethanol
B) acetonitrile
C) dimethylformamide
D) acetone
E) 18-crown-6
A) ethanol
B) acetonitrile
C) dimethylformamide
D) acetone
E) 18-crown-6

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
49
List the following alkyl halides in order of their increasing reactivity as substrates in SN2 reactions: 2-chlorobutane, 2-iodobutane, and 1-iodobutane.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following alkyl chlorides would undergo substitution most rapidly when treated with 
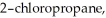
or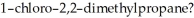
Provide the structure of the substitution product.

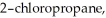
or
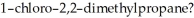
Provide the structure of the substitution product.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
51
Provide the structure of the major organic product in the following reaction.
(CH3)3N + CH3CH2CH2I →
(CH3)3N + CH3CH2CH2I →

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following alkyl chlorides will undergo SN2 reaction most readily?
A) 1-chloro-4-methylpentane
B) 2-chloro-4-methylpentane
C) 2-chloro-3-methylpentane
D) 3-chloro-2-methylpentane
E) 2-chloro-2-methylpentane
A) 1-chloro-4-methylpentane
B) 2-chloro-4-methylpentane
C) 2-chloro-3-methylpentane
D) 3-chloro-2-methylpentane
E) 2-chloro-2-methylpentane

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following compounds is the most nucleophilic?
A) CH3SH
B) CH3OH
C) H2O
D) CH3CO2H
E) BF3
A) CH3SH
B) CH3OH
C) H2O
D) CH3CO2H
E) BF3

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
54
Consider the substitution reaction shown below. By what mechanism will the reaction proceed? 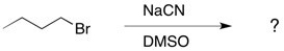
A) SN1
B) SN2
C) free radical reaction
D) There is not enough information to tell.

A) SN1
B) SN2
C) free radical reaction
D) There is not enough information to tell.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
55
What type of solvent is best for SN2 reactions which employ anionic nucleophiles: polar, protic solvents; polar, aprotic solvents; or nonpolar solvents? Explain.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following factors is favorable for nucleophilicity but not basicity?
A) highly polarizable
B) low electronegativity
C) negatively charged
D) sterically hindered
A) highly polarizable
B) low electronegativity
C) negatively charged
D) sterically hindered

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
57
Name one factor that is favorable for nucleophilicity but not for basicity.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
58
Which is more nucleophilic, t-butoxide or ethoxide? Explain.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
59
Do all primary alkyl iodides undergo SN2 reactions with sodium cyanide in DMSO at identical rates? Explain.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following species is the least nucleophilic?
A) H2O
B) BF3
C) (CH3)3N
D) CH3O-
E) CN-
A) H2O
B) BF3
C) (CH3)3N
D) CH3O-
E) CN-

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the compounds below undergoes solvolysis in aqueous ethanol most rapidly?
A) cyclohexyl bromide
B) methyl iodide
C) isopropyl chloride
D) 3-chloropentane
E) 3-iodo-3-methylpentane
A) cyclohexyl bromide
B) methyl iodide
C) isopropyl chloride
D) 3-chloropentane
E) 3-iodo-3-methylpentane

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
62
Provide the structure of the major organic product which results when (S)-2-iodopentane is treated with KCN in DMF.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following alkyl bromides undergoes solvolysis in aqueous methanol most rapidly?
A) PhCHBrCH3
B) (CH3)2CHCH2CH2Br
C) (CH3)2CHCH2CHBrCH3
D) CH3CH2CH2CH2Br
E) PhBr
A) PhCHBrCH3
B) (CH3)2CHCH2CH2Br
C) (CH3)2CHCH2CHBrCH3
D) CH3CH2CH2CH2Br
E) PhBr

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
64
List the following bromides in order of their increasing reactivity as substrates in SN1 reactions: 1-iodo-1-ethylcyclopentane, chlorocyclopentane, and iodocyclopentane.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
65
List the following compounds in order of increasing reactivity in an SN1 reaction.
CH3Br, CH3CH2CH2I, (CH3)3CI, CH3CHBrCH3, CH3CHICH3
CH3Br, CH3CH2CH2I, (CH3)3CI, CH3CHBrCH3, CH3CHICH3

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
66
Draw the highest energy transition state in the solvolysis of (CH3)3CI in CH3OH.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
67
SN2 reactions take place with ________ of stereochemistry at the center undergoing substitution.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
68
Rank the following molecules in order of increasing relative rate of SN1 solvolysis with methanol and heat (slowest to fastest reacting). 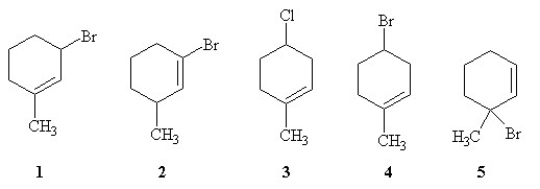
A) 3 < 2 < 4 < 5 < 1
B) 2 < 3 < 4 < 1 < 5
C) 5 < 4 < 3 < 2 < 1
D) 2 < 3 < 4 < 5 < 1
E) 1 < 2 < 5 < 4 < 3

A) 3 < 2 < 4 < 5 < 1
B) 2 < 3 < 4 < 1 < 5
C) 5 < 4 < 3 < 2 < 1
D) 2 < 3 < 4 < 5 < 1
E) 1 < 2 < 5 < 4 < 3

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
69
List the following bromides in order of their increasing reactivity as substrates in SN1 reactions: PhBr, PhCH2Br, and PhCH(CH3)Br.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
70
Provide the major organic product of the reaction below. 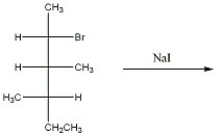


Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
71
Provide the major organic product of the reaction below. 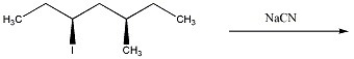


Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following alkyl halides reacts most rapidly via an SN1 solvolysis reaction in hot methanol?
A) 1-iodohexane
B) 1-fluorohexane
C) (R)-2-bromohexane
D) iodocyclohexane
E) 1-iodo-1-methylcyclohexane
A) 1-iodohexane
B) 1-fluorohexane
C) (R)-2-bromohexane
D) iodocyclohexane
E) 1-iodo-1-methylcyclohexane

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
73
Show the best way to prepare CH3OCH(CH3)2 by an SN2 reaction.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
74
Provide the major organic product of the SN2 reaction below. 


Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following statements describe a favorable attribute of a leaving group? (More than one answer is possible.)
A) The leaving group should be highly polarizable.
B) The lower the electronegativity of the leaving group the better.
C) Good leaving groups need to have low steric hindrance.
D) The departed leaving group should have low basicity.
A) The leaving group should be highly polarizable.
B) The lower the electronegativity of the leaving group the better.
C) Good leaving groups need to have low steric hindrance.
D) The departed leaving group should have low basicity.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
76
Complete the following SN2 reaction by providing structures for the necessary starting materials. 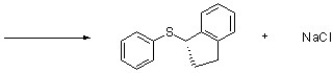


Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
77
List the following bromides in order of their increasing reactivity as substrates in SN1 reactions: 2-chlorobutane, 2-iodobutane, and 1-iodobutane.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following alkyl halides reacts most rapidly via an SN2 reaction with NaCN?
A) 1-iodohexane
B) 1-fluorohexane
C) (R)-2-bromohexane
D) iodocyclohexane
E) 1-iodo-1-methylcyclohexane
A) 1-iodohexane
B) 1-fluorohexane
C) (R)-2-bromohexane
D) iodocyclohexane
E) 1-iodo-1-methylcyclohexane

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
79
Provide the structure of the major organic product in the following reaction. 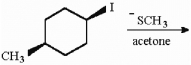


Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
80
Arrange the following compounds in order of increasing reactivity toward ethanol solvolysis:
t-butyl bromide, t-butyl iodide, isopropyl chloride, methyl iodide.
t-butyl bromide, t-butyl iodide, isopropyl chloride, methyl iodide.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck



