Deck 18: Ketones and Aldehydes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/131
Play
Full screen (f)
Deck 18: Ketones and Aldehydes
1
Provide the structure of benzophenone.
PhCOPh
2
Provide the proper IUPAC name for CH3CHBrCH2COCH2CHO.
5-bromo-3-oxohexanal
3
Provide the IUPAC name for (CH3)3CCH2CHClCH2CHO.
3-chloro-5,5-dimethylhexanal
4
Name the following compound. 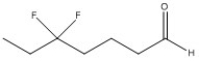


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
5
The positively polarized carbon atom of a carbonyl group acts as ________.
A) an electrophile and a Lewis base
B) a nucleophile and a Lewis base
C) an electrophile and a Lewis acid
D) a nucleophile and a Lewis acid
E) both a Lewis acid and a Lewis base
A) an electrophile and a Lewis base
B) a nucleophile and a Lewis base
C) an electrophile and a Lewis acid
D) a nucleophile and a Lewis acid
E) both a Lewis acid and a Lewis base

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
6
Provide the structure of 4-oxohexanoic acid.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
7
Would you expect the carbonyl carbon of benzaldehyde to be more or less electrophilic than that of acetaldehyde? Explain using resonance structures.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
8
Name the following compound. 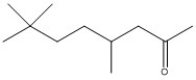


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
9
Provide the proper IUPAC name for CH3OCH2CH2CH(CH3)CHO.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
10
Provide the proper IUPAC name for CH3CHOHCH2COCH2C(CH3)2CH2CH3.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following correctly describes the bond angle and hybridizations present in formaldehyde?
A) C, sp2; O, sp3; HCO, ~120°
B) C, sp2; O, sp2; HCO, ~120°
C) C, sp2; O, sp2; HCO, ~109.5°
D) C, sp3; O, sp2; HCO, ~109.5°
E) C, sp3; O, sp3; HCO, ~109.5°
A) C, sp2; O, sp3; HCO, ~120°
B) C, sp2; O, sp2; HCO, ~120°
C) C, sp2; O, sp2; HCO, ~109.5°
D) C, sp3; O, sp2; HCO, ~109.5°
E) C, sp3; O, sp3; HCO, ~109.5°

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
12
Provide the proper IUPAC name for (CH3)2CHCH2CH2COCH 
CH2.

CH2.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
13
Give an IUPAC name to the following compound. Be sure to include configurational information in the name. 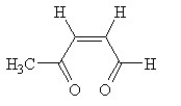


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
14
Provide the structure of cyclohexanecarbaldehyde.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
15
The structure of Taxol, a potent chemotherapeutic, is shown below. In the structure, there are ________ ketones and ________ aldehydes. 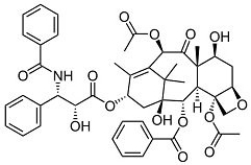
A) 1 ketone; 1 aldehyde
B) 1 ketone; 0 aldehydes
C) 6 ketones; 0 aldehydes
D) 5 ketones; 1 aldehyde
E) 0 ketones; 0 aldehydes

A) 1 ketone; 1 aldehyde
B) 1 ketone; 0 aldehydes
C) 6 ketones; 0 aldehydes
D) 5 ketones; 1 aldehyde
E) 0 ketones; 0 aldehydes

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
16
Provide the IUPAC name for the following compound, including stereochemical designators as appropriate. 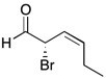


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
17
What is the correct IUPAC name for the following compound? 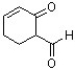
A) 2-oxocyclohex-3-ene carbaldehyde
B) 5-formylcyclohex-2-enone
C) 2-formylcyclohex-5-enone
D) 3-oxocyclohex-4-enal

A) 2-oxocyclohex-3-ene carbaldehyde
B) 5-formylcyclohex-2-enone
C) 2-formylcyclohex-5-enone
D) 3-oxocyclohex-4-enal

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
18
Provide the IUPAC name for the following compound. 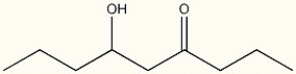


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
19
Another name for β-methoxybutyraldehyde is ________.
A) 2-methoxypropanal
B) 3-methoxypropanal
C) 2-methoxybutanal
D) 3-methoxybutanal
E) 2-methoxypentanal
A) 2-methoxypropanal
B) 3-methoxypropanal
C) 2-methoxybutanal
D) 3-methoxybutanal
E) 2-methoxypentanal

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
20
Provide the proper IUPAC name for PhCH2CH(CH3)CH2CH2CHO.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
21
In the proton NMR spectra of aldehydes and ketones, the protons bonded to carbons adjacent to the carbonyl group typically fall into which of the chemical shift ranges below?
A) 1.0-2.0 ppm
B) 2.0-3.0 ppm
C) 4.0-4.5 ppm
D) 7.0-8.0 ppm
E) 9.0-10.0 ppm
A) 1.0-2.0 ppm
B) 2.0-3.0 ppm
C) 4.0-4.5 ppm
D) 7.0-8.0 ppm
E) 9.0-10.0 ppm

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
22
Predict the major organic product of the following reaction sequence. 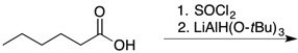
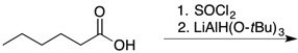

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
23
Provide the major organic product which results when PhCHOHCH3 is treated with PCC.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
24
In the mass spectrum of 3,3-dimethyl-2-butanone, the base peak will occur at m/z ________.
A) 43
B) 58
C) 84
D) 85
E) 100
A) 43
B) 58
C) 84
D) 85
E) 100

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following compounds would show only one triplet in its off resonance decoupled  spectrum?
spectrum?
A) acetone
B) butanal
C) pentanal
D) 2-pentanone
E) 3-pentanone
 spectrum?
spectrum?A) acetone
B) butanal
C) pentanal
D) 2-pentanone
E) 3-pentanone

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
26
How might one distinguish an aldehyde from a ketone using IR data alone?

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
27
In carbon NMR, the carbon atom of the carbonyl group in aldehydes and ketones has a chemical shift of about ________.
A) 20 ppm
B) 40 ppm
C) 60 ppm
D) 120 ppm
E) 200 ppm
A) 20 ppm
B) 40 ppm
C) 60 ppm
D) 120 ppm
E) 200 ppm

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
28
The strongest absorptions in the UV spectra of aldehydes and ketones are ones which result from ________ electronic transitions.
A) σ to σ*
B) σ to π*
C) n to π*
D) π to π*
E) π to σ*
A) σ to σ*
B) σ to π*
C) n to π*
D) π to π*
E) π to σ*

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following transitions is usually observed in the UV spectra of ketones?
A) n to π*
B) n to π
C) σ to n
D) σ to σ*
E) n to σ*
A) n to π*
B) n to π
C) σ to n
D) σ to σ*
E) n to σ*

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
30
The proton NMR spectrum of an unknown compound contains a triplet at 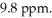 Which of the following could be this unknown?
Which of the following could be this unknown?
A) (CH3)3CCHO
B) CH3CH2CH2CO2H
C) CH3CH2CH2CHO
D) CH3CO CH2Ph
E) PhCHO
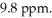 Which of the following could be this unknown?
Which of the following could be this unknown?A) (CH3)3CCHO
B) CH3CH2CH2CO2H
C) CH3CH2CH2CHO
D) CH3CO CH2Ph
E) PhCHO

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following compounds is most soluble in water?
A) acetone
B) cyclohexanone
C) 2-butanone
D) 3-octanone
E) benzophenone
A) acetone
B) cyclohexanone
C) 2-butanone
D) 3-octanone
E) benzophenone

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
32
Name the following compound. 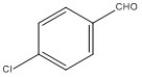


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following represents the correct ranking in terms of increasing boiling point?
A) n-butane < 1-butanol < diethyl ether < 2-butanone
B) n-butane < 2-butanone < diethyl ether < 1-butanol
C) 2-butanone < n-butane < diethyl ether < 1-butanol
D) n-butane < diethyl ether < 1-butanol < 2-butanone
E) n-butane < diethyl ether < 2-butanone < 1-butanol
A) n-butane < 1-butanol < diethyl ether < 2-butanone
B) n-butane < 2-butanone < diethyl ether < 1-butanol
C) 2-butanone < n-butane < diethyl ether < 1-butanol
D) n-butane < diethyl ether < 1-butanol < 2-butanone
E) n-butane < diethyl ether < 2-butanone < 1-butanol

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
34
Provide the major organic product which results when PhCHO is treated with the following sequence of reagents: 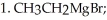
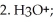
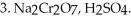
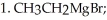
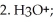
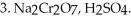

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
35
When a carbonyl is part of a conjugated π-network, the C  O stretch ________.
O stretch ________.
A) has a higher frequency than in a nonconjugated system
B) has a lower frequency than in a nonconjugated system
C) always occurs at 1710 cm-1
D) occurs around 2700 cm-1
E) cannot be distinguished from the C O stretch in a nonconjugated system
O stretch in a nonconjugated system
 O stretch ________.
O stretch ________.A) has a higher frequency than in a nonconjugated system
B) has a lower frequency than in a nonconjugated system
C) always occurs at 1710 cm-1
D) occurs around 2700 cm-1
E) cannot be distinguished from the C
 O stretch in a nonconjugated system
O stretch in a nonconjugated system
Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
36
Which is more soluble in water, 2-butanone or n-hexane? Explain.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
37
How would you perform the following transformation? 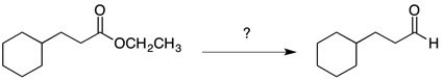
A) NaBH4
B) LiAlH4
C) 1. DIBAL-H 2. H2O
D) 1. SOCl2 2. LiAlH(O-t-Bu)3
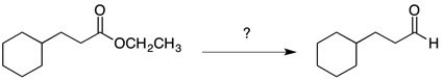
A) NaBH4
B) LiAlH4
C) 1. DIBAL-H 2. H2O
D) 1. SOCl2 2. LiAlH(O-t-Bu)3

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
38
What reagent can be used to convert 2-methylbutan-1-ol into 2-methylbutanal?
A) LiAlH4
B) Na2Cr2O7
C) O3
D) KMnO4
E) PCC
A) LiAlH4
B) Na2Cr2O7
C) O3
D) KMnO4
E) PCC

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
39
What reagents can be used to convert 1-hexyne into 2-hexanone?
A) 1. Sia2BH; 2. H2O2, NaOH
B) Hg2+, H2SO4, H2O
C) 1. O3; 2. (CH3)2S
D) 1. CH3MgBr; 2. CO2
E) 1. H2, Ni; 2. Na2Cr2O7, H2SO4
A) 1. Sia2BH; 2. H2O2, NaOH
B) Hg2+, H2SO4, H2O
C) 1. O3; 2. (CH3)2S
D) 1. CH3MgBr; 2. CO2
E) 1. H2, Ni; 2. Na2Cr2O7, H2SO4

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
40
Which compound will show an intense peak in the mass spectrum at m/z 58?
A) CH3COCH2CH2CH3
B) (CH3)2CHCOCH3
C) CH3CH2COCH2CH3
D) (CH3)3CCHO
E) (CH3)3CCOCH3
A) CH3COCH2CH2CH3
B) (CH3)2CHCOCH3
C) CH3CH2COCH2CH3
D) (CH3)3CCHO
E) (CH3)3CCOCH3

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
41
Which series of reactions described below, if any, will result in the formation of 2-methylpentan-3-one starting with 1-propanol?
A) 1. (CH3)2CHMgBr/ diethyl ether
2) dilute H3O+
3) PCC
B) 1. Na2Cr2O7/H2SO4 and heat
2) SOCl2
3) 2 (CH3)2CHMgBr/ diethyl ether
4) H3O+
C) 1. Na2Cr2O7/H2SO4 and heat
2) (CH3)2CHMgBr/ diethyl ether
3) dilute H3O+
4) LiAlH4
D) 1. PCC
2) (CH3)2CHLi/ diethyl ether
3) dilute H3O+
4) Na2Cr2O7/H2SO4 and heat
E) none of the above
A) 1. (CH3)2CHMgBr/ diethyl ether
2) dilute H3O+
3) PCC
B) 1. Na2Cr2O7/H2SO4 and heat
2) SOCl2
3) 2 (CH3)2CHMgBr/ diethyl ether
4) H3O+
C) 1. Na2Cr2O7/H2SO4 and heat
2) (CH3)2CHMgBr/ diethyl ether
3) dilute H3O+
4) LiAlH4
D) 1. PCC
2) (CH3)2CHLi/ diethyl ether
3) dilute H3O+
4) Na2Cr2O7/H2SO4 and heat
E) none of the above

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
42
Beginning with sodium acetylide (NaCCH), propose a three-step synthesis of hexanal.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following describes a synthesis of an aldehyde?
A) hydrogenation of an acid chloride using Pd/BaSO4/S as a poisoned catalyst
B) reaction of a primary alcohol with Na2Cr2O7
C) reaction of a ketone with ozone
D) treatment of an alkene with Sia2BH
E) none of the above
A) hydrogenation of an acid chloride using Pd/BaSO4/S as a poisoned catalyst
B) reaction of a primary alcohol with Na2Cr2O7
C) reaction of a ketone with ozone
D) treatment of an alkene with Sia2BH
E) none of the above

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
44
Provide the major organic product which results when 
is treated with excess butyllithium followed by H3O+.
is treated with excess butyllithium followed by H3O+.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
45
Complete the following reaction by filling in the necessary reagents. 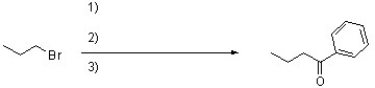


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
46
Show how you would perform the following synthesis. 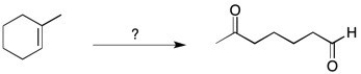
A) 1. O3 2. (CH3)2S
B) KMnO4, cold, basic
C) OsO4
D) 1. mCPBA 2. potassium dichromate

A) 1. O3 2. (CH3)2S
B) KMnO4, cold, basic
C) OsO4
D) 1. mCPBA 2. potassium dichromate

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
47
Name the compound generated when ethylbenzene is treated with CO, HCl, AlCl3, and CuCl.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
48
Oxidation of a 1° alcohol with pyridinium chlorochromate results in the production of ________.
A) an ester
B) a ketone
C) an aldehyde
D) an ether
E) none of the above
A) an ester
B) a ketone
C) an aldehyde
D) an ether
E) none of the above

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
49
Provide the major organic product of the reaction shown below. 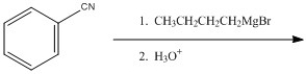


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
50
Provide the major organic product which results when pentanal is subjected to the following sequence of steps: 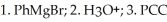
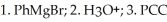

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
51
Oxidation of a 2° alcohol with chromic acid results in the production of ________.
A) an ester
B) a ketone
C) an aldehyde
D) an ether
E) none of the above
A) an ester
B) a ketone
C) an aldehyde
D) an ether
E) none of the above

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
52
Provide the major organic product of the reaction shown below. 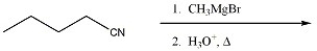
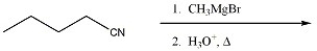

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
53
By which single reaction can benzene be readily converted into acetophenone?

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
54
Provide the reagents to perform the following synthesis. 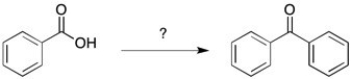


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
55
Treatment of a nitrile with a Grignard reagent followed by hydrolysis results in ________.
A) an ester
B) a ketone
C) an aldehyde
D) an ether
E) an alcohol
A) an ester
B) a ketone
C) an aldehyde
D) an ether
E) an alcohol

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
56
Propose a synthesis of 3-heptanone from propanal.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
57
Oxidation of a 3° alcohol with chromic acid results in the production of ________.
A) an ester
B) a ketone
C) an aldehyde
D) an ether
E) none of the above
A) an ester
B) a ketone
C) an aldehyde
D) an ether
E) none of the above

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following reactions will not yield a ketone product?
A)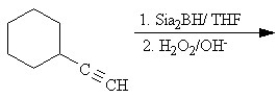
B)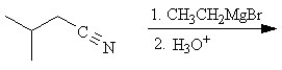
C)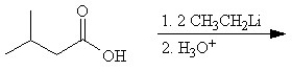
D)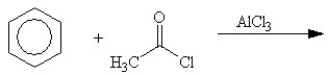
E)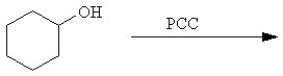
A)

B)
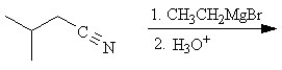
C)
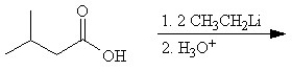
D)

E)
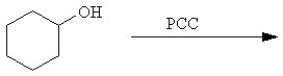

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
59
Show how you would perform the following synthesis. 


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
60
How might one prepare 4-heptanone from CH3CH2CH2CN in two steps?

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
61
Provide the structure of the hydrate of cyclopentanone.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
62
What organic compound is generated when PhCOCl is treated with 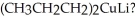
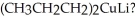

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
63
When the carbonyl group of a neutral ketone is protonated ________.
A) the resulting species becomes more electrophilic
B) the resulting species is activated toward nucleophilic attack
C) subsequent nucleophilic attack on the resulting species is said to occur under acid-catalyzed conditions
D) the resulting species has a positive charge
E) all of the above
A) the resulting species becomes more electrophilic
B) the resulting species is activated toward nucleophilic attack
C) subsequent nucleophilic attack on the resulting species is said to occur under acid-catalyzed conditions
D) the resulting species has a positive charge
E) all of the above

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
64
Provide the major organic product of the reaction shown below. 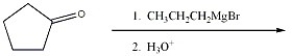
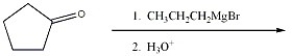

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
65
Provide the major organic product of the reaction shown below. 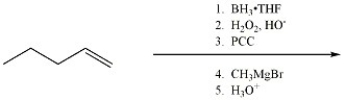
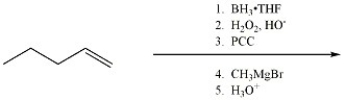

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
66
Provide the major organic product of the reaction shown below. 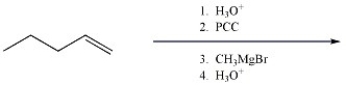


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
67
Provide the single reagent necessary for the conversion of cyclobutanone to the compound shown below. 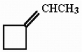


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
68
An ylide is a molecule that can be described as a ________.
A) carbanion bound to a negatively charged heteroatom
B) carbocation bound to a positively charged heteroatom
C) carbocation bound to a carbon radical
D) carbocation bound to a diazonium ion
E) carbanion bound to a positively charged heteroatom
A) carbanion bound to a negatively charged heteroatom
B) carbocation bound to a positively charged heteroatom
C) carbocation bound to a carbon radical
D) carbocation bound to a diazonium ion
E) carbanion bound to a positively charged heteroatom

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
69
Propose a synthesis of 4-phenylbutan-2-ol from 3-phenylpropanal.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
70
Propose a synthesis of 3-methylhept-4-yn-3-ol from but-1-yne.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
71
Provide a detailed, stepwise mechanism for the base-catalyzed hydration of 2-butanone.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
72
Provide the major organic product which results when 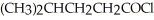
is treated with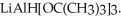
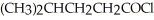
is treated with
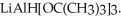

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
73
The following compound has been found effective in treating pain and inflammation (J. Med. Chem. 2007, 4222). Which sequence correctly ranks each carbonyl group in order of increasing reactivity toward nucleophilic addition? 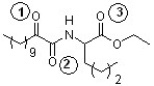
A) 1 < 2 < 3
B) 2 < 3 < 1
C) 3 < 1 < 2
D) 1 < 3 < 2

A) 1 < 2 < 3
B) 2 < 3 < 1
C) 3 < 1 < 2
D) 1 < 3 < 2

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
74
Which sequence ranks the following carbonyl compounds in order of increasing rate of nucleophilic addition? 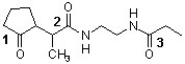
A) 2 < 3 < 1
B) 3 < 2 < 1
C) 2 < 1 < 3
D) 1 < 3 < 2

A) 2 < 3 < 1
B) 3 < 2 < 1
C) 2 < 1 < 3
D) 1 < 3 < 2

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
75
Why are the equilibrium constants for hydration of aldehydes typically greater than those of ketones?

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
76
Consider the equilibrium of each of the carbonyl compounds with HCN to produce cyanohydrins. Which is the correct ranking of compounds in order of increasing Keq for this equilibrium?
A) H2CO < cyclohexanone < CH3CHO < 2-methylcyclohexanone
B) CH3CHO < 2-methylcyclohexanone < cyclohexanone < H2CO
C) cyclohexanone < 2-methylcyclohexanone < H2CO < CH3CHO
D) cyclohexanone < 2-methylcyclohexanone < CH3CHO < H2CO
E) 2-methylcyclohexanone < cyclohexanone < CH3CHO < H2CO
A) H2CO < cyclohexanone < CH3CHO < 2-methylcyclohexanone
B) CH3CHO < 2-methylcyclohexanone < cyclohexanone < H2CO
C) cyclohexanone < 2-methylcyclohexanone < H2CO < CH3CHO
D) cyclohexanone < 2-methylcyclohexanone < CH3CHO < H2CO
E) 2-methylcyclohexanone < cyclohexanone < CH3CHO < H2CO

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
77
Draw two major resonance forms of the cation which results when cyclohexanone's carbonyl group is protonated.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
78
Rank the following compounds in order of their propensity to become a hydrate in water (i.e., start with the least easy to hydrate: CH3COCH2CH3, H2CO, Cl3CCHO, and CH3CH2CHO.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
79
What reagent can be used to convert benzophenone into triphenylmethanol?

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
80
Through what sequence of steps can toluene be converted into 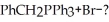
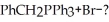

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck



