Deck 6: Chemical Reactivity and Mechanisms
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/86
Play
Full screen (f)
Deck 6: Chemical Reactivity and Mechanisms
1
Which of the following is the enthalpy of the following reaction? 
A)-8 kJ/mol
B)8 kJ/mol
C)-506 kJ/mol
D)63 kJ/mol

A)-8 kJ/mol
B)8 kJ/mol
C)-506 kJ/mol
D)63 kJ/mol
-8 kJ/mol
2
Calculate the enthalpy of the following reaction. 

29 kJ/mol
3
Does a reaction with a positive S and a negative H favor reactants or products?
Products
4
Calculate the enthalpy of the following reaction. 


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
5
Calculate the enthalpy of the following reaction. 


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is the enthalpy of the following reaction? 
A)-8 kJ/mol
B)8 kJ/mol
C)222 kJ/mol
D)79 kJ/mol

A)-8 kJ/mol
B)8 kJ/mol
C)222 kJ/mol
D)79 kJ/mol

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
7
What type of cleavage does the following reaction involve? 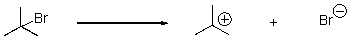
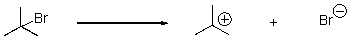

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
8
Predict the sign of G for an endothermic reaction with an increase in entropy.
A)positive
B)negative
C)no change
D)cannot predict
A)positive
B)negative
C)no change
D)cannot predict

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
9
Predict the sign of S of the following reaction? 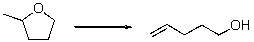


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
10
What type of cleavage does the following reaction involve? 
A)homolytic
B)heterolytic

A)homolytic
B)heterolytic

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
11
Does a reaction with a negative G favor reactants or products?

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
12
Predict the sign of S of the following reaction? 
A)positive
B)negative
C)no change
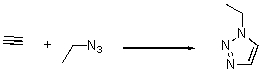
A)positive
B)negative
C)no change

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
13
Predict the sign of G for an endothermic reaction with a decrease in entropy.
A)positive
B)negative
C)no change
D)cannot predict
A)positive
B)negative
C)no change
D)cannot predict

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
14
Does a reaction with a positive G favor reactants or products?

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
15
Predict the sign of S of the following reaction? 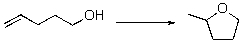
A)positive
B)negative
C)no change
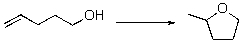
A)positive
B)negative
C)no change

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
16
Predict the sign of G for an exothermic reaction with a decrease in entropy.
A)positive
B)negative
C)no change
D)cannot predict
A)positive
B)negative
C)no change
D)cannot predict

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
17
Does a reaction with a positive S and a positive H favor reactants or products?

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
18
Predict the sign of G for an exothermic reaction with an increase in entropy.
A)positive
B)negative
C)no change
D)cannot predict
A)positive
B)negative
C)no change
D)cannot predict

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
19
Predict the sign of S of the following reaction? 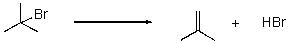
A)positive
B)negative
C)no change
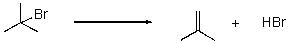
A)positive
B)negative
C)no change

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
20
Why is entropy negative for ring closures?
A)Closing a ring results in fewer molecules.
B)Closing a ring results in more molecules.
C)Closing a ring releases energy.
D)Closing a ring restricts the rotation around individual carbon-carbon bonds.
A)Closing a ring results in fewer molecules.
B)Closing a ring results in more molecules.
C)Closing a ring releases energy.
D)Closing a ring restricts the rotation around individual carbon-carbon bonds.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
21
Given the following rate law,the reaction is what order in tert-butyliodide? 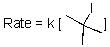
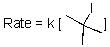

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
22
Identify the nucleophilic atom in the following molecule. 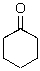


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
23
Identify the nucleophilic atom in the following molecule. 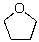


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
24
Based on the following energy diagram,is the following reaction exothermic or endothermic? 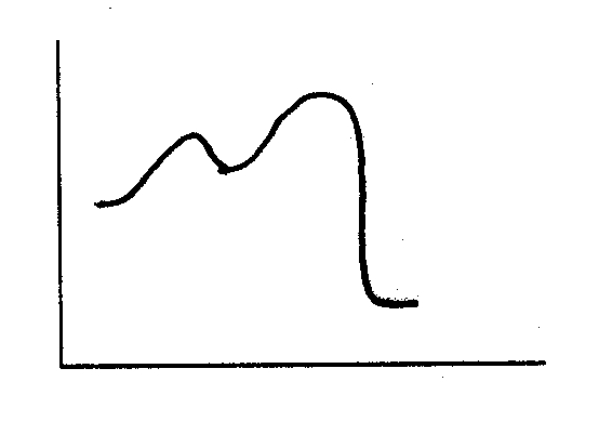


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
25
Does a reaction with a H of 14 kJ/mol and a S of 150 J/molK at 298 K favor reactants or products?

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
26
What is the energy of activation for the following reaction? 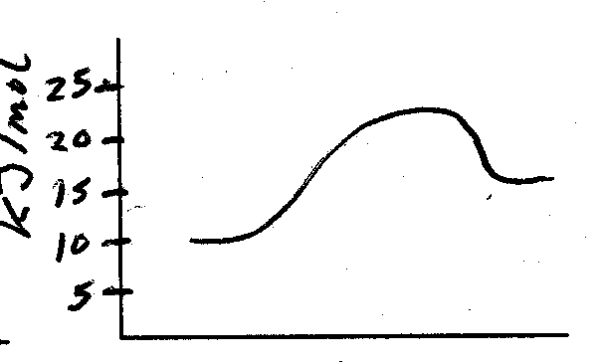


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
27
Draw an energy diagram for an endothermic reaction with two steps.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
28
What is the effect of a catalyst on a reaction?
A)It speeds up the rate.
B)It decreases the entropy.
C)It changes the equilibrium.
D)It makes the products more stable.
A)It speeds up the rate.
B)It decreases the entropy.
C)It changes the equilibrium.
D)It makes the products more stable.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
29
Given the following rate law,the reaction is what order in sodium cyanide? 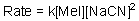
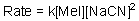

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
30
Given the following rate law,the reaction is what order in MeI? 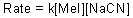
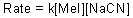

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
31
Identify the nucleophilic atom(s)in the following molecule. 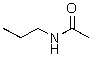


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
32
What is the energy of activation for the following reaction? 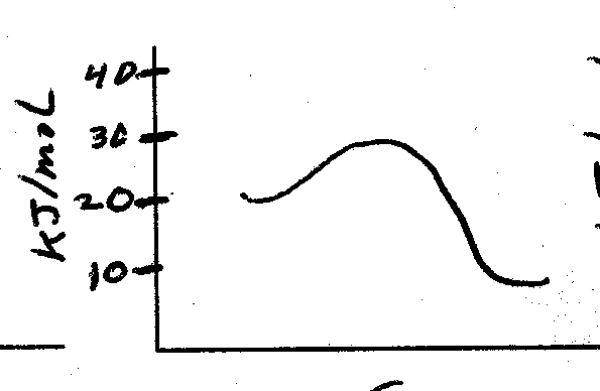


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following describes the effect of a catalyst on a reaction?
A)It lowers the free energy of the products.
B)It makes the reactants less stable.
C)It changes the equilibrium constant.
D)It lowers the energy of activation.
A)It lowers the free energy of the products.
B)It makes the reactants less stable.
C)It changes the equilibrium constant.
D)It lowers the energy of activation.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
34
Draw an energy diagram for a three step reaction.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
35
Based on the following energy diagram,is the following reaction exothermic or endothermic? 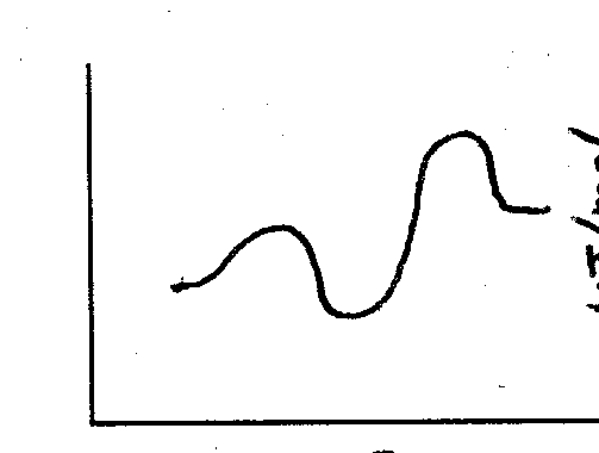


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
36
Does a reaction with a Keq = 10 favor reactants or products?

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
37
Draw an energy diagram for an exothermic concerted reaction.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
38
What is a transition state?
A)An isolable intermediate in a reaction.
B)The starting materials of the reaction.
C)A local maximum on the energy diagram.
D)A point between the starting materials and the product.
A)An isolable intermediate in a reaction.
B)The starting materials of the reaction.
C)A local maximum on the energy diagram.
D)A point between the starting materials and the product.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
39
Does a reaction with a H or 20 kJ/mol and a S of 10 J/mol K at 298 K favor reactants of products?

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
40
What is an intermediate?
A)A local maximum on the energy diagram.
B)A point on the reaction pathway that has a discrete lifetime.
C)A point half-way between the starting materials and products.
D)The highest energy compound on an energy diagram.
A)A local maximum on the energy diagram.
B)A point on the reaction pathway that has a discrete lifetime.
C)A point half-way between the starting materials and products.
D)The highest energy compound on an energy diagram.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
41
Draw the structure for hydride.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
42
For the following reaction step,indicate which pattern it belongs to. 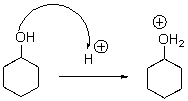
A)Proton Transfer
B)Loss of Leaving Group
C)Nucleophilic Attack
D)Rearrangement

A)Proton Transfer
B)Loss of Leaving Group
C)Nucleophilic Attack
D)Rearrangement

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
43
For the following reaction step,indicate which pattern it belongs to. 
A)Proton Transfer
B)Loss of Leaving Group
C)Nucleophilic Attack
D)Rearrangement

A)Proton Transfer
B)Loss of Leaving Group
C)Nucleophilic Attack
D)Rearrangement

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
44
For the following reaction step,indicate which pattern it belongs to. 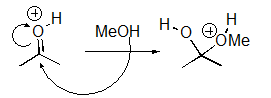


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following is hydride?
A)H+
B)H
C)H-
D)H2
A)H+
B)H
C)H-
D)H2

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
46
For the following reaction step,indicate which pattern it belongs to. 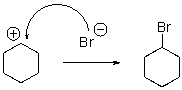
A)Proton Transfer
B)Loss of Leaving Group
C)Nucleophilic Attack
D)Rearrangement

A)Proton Transfer
B)Loss of Leaving Group
C)Nucleophilic Attack
D)Rearrangement

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
47
Identify the electrophilic site in the following molecule. 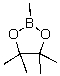
A)C
B)O
C)B
D)No electrophilic site

A)C
B)O
C)B
D)No electrophilic site

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
48
Identify the electrophilic atom in the following molecule. 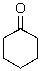


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
49
For the following reaction step,indicate which pattern it belongs to. 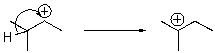
A)Proton Transfer
B)Loss of Leaving Group
C)Nucleophilic Attack
D)Rearrangement

A)Proton Transfer
B)Loss of Leaving Group
C)Nucleophilic Attack
D)Rearrangement

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
50
Identify the nucleophilic atom in the following molecule. 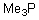
A)P
B)C
C)H
D)Me
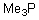
A)P
B)C
C)H
D)Me

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
51
What is wrong with the following mechanism? 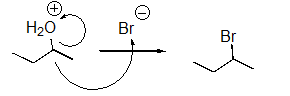
A)There is no leaving group,so there should be no arrows.
B)The arrow should be removing a proton from the H2O group.
C)An arrow is also needed to indicate the loss of the leaving group.
D)The arrow indicating the formation of the C-Br bond (nucleophilic attack)should start at the bromide anion.

A)There is no leaving group,so there should be no arrows.
B)The arrow should be removing a proton from the H2O group.
C)An arrow is also needed to indicate the loss of the leaving group.
D)The arrow indicating the formation of the C-Br bond (nucleophilic attack)should start at the bromide anion.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
52
Identify the electrophilic site in the following molecule. 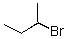


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
53
Identify the nucleophilic centers in the following molecule. 


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
54
Identify the electrophilic site in the following molecule. 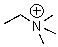


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
55
For the following reaction step,indicate which pattern it belongs to. 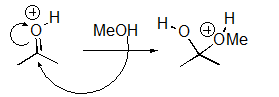
A)Proton Transfer
B)Loss of Leaving Group
C)Nucleophilic Attack
D)Rearrangement

A)Proton Transfer
B)Loss of Leaving Group
C)Nucleophilic Attack
D)Rearrangement

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
56
For the following reaction step,indicate which pattern it belongs to. 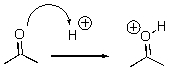
A)Proton Transfer
B)Loss of Leaving Group
C)Nucleophilic Attack
D)Rearrangement

A)Proton Transfer
B)Loss of Leaving Group
C)Nucleophilic Attack
D)Rearrangement

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
57
What is wrong with the following mechanism? 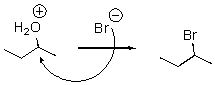
A)There is no leaving group,so there should be no arrows.
B)The arrow should be removing a proton from the H2O group.
C)An arrow is also needed to indicate the loss of the leaving group.
D)The arrow is backwards.

A)There is no leaving group,so there should be no arrows.
B)The arrow should be removing a proton from the H2O group.
C)An arrow is also needed to indicate the loss of the leaving group.
D)The arrow is backwards.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
58
Identify the nucleophilic centers in the following molecule. 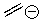
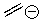

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
59
Identify the electrophilic atom in the following molecule. 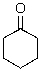
A)C
B)H
C)O of carbonyl
D)C of carbonyl

A)C
B)H
C)O of carbonyl
D)C of carbonyl

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
60
Identify the electrophilic site in the following molecule. 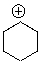


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
61
What pattern is the second step of this reaction? 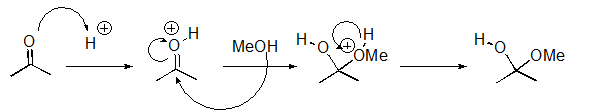


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
62
Identify the sequence of steps in the following reaction. 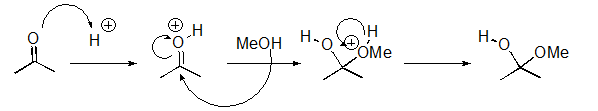
A)Proton transfer,Proton transfer,Nucleophilic Attack
B)Proton transfer,Loss of leaving group
C)Nucleophilic attack,Proton transfer,Loss of leaving group
D)Proton transfer,Nucleophilic attack,Proton transfer

A)Proton transfer,Proton transfer,Nucleophilic Attack
B)Proton transfer,Loss of leaving group
C)Nucleophilic attack,Proton transfer,Loss of leaving group
D)Proton transfer,Nucleophilic attack,Proton transfer

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
63
Will the following cation undergo rearrangement? 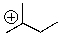


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following is the nucleophilic site in the following reaction? 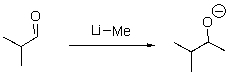
A)C of the carbonyl
B)O
C)Li
D)Me of Li-Me

A)C of the carbonyl
B)O
C)Li
D)Me of Li-Me

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
65
Identify the sequence of steps in the following reaction. 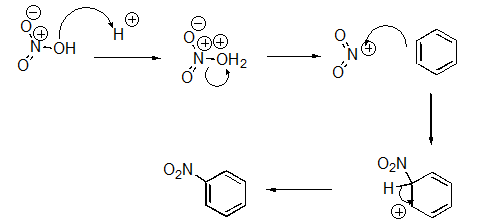
A)Proton transfer,Proton transfer,Nucleophilic Attack
B)Proton transfer,Loss of leaving group,Nucleophilic attack,Proton transfer
C)Nucleophilic attack,Proton transfer,Loss of leaving group
D)Proton transfer,Loss of leaving group,Proton transfer,Nucleophilic attack

A)Proton transfer,Proton transfer,Nucleophilic Attack
B)Proton transfer,Loss of leaving group,Nucleophilic attack,Proton transfer
C)Nucleophilic attack,Proton transfer,Loss of leaving group
D)Proton transfer,Loss of leaving group,Proton transfer,Nucleophilic attack

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
66
Identify the nucleophilic and electrophilic sites in the following reaction. 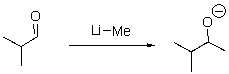


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
67
What pattern is the second step of this reaction? 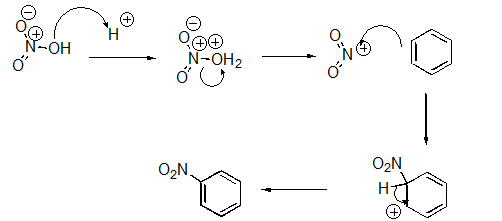
A)Proton Transfer
B)Loss of Leaving Group
C)Nucleophilic Attack
D)Rearrangement

A)Proton Transfer
B)Loss of Leaving Group
C)Nucleophilic Attack
D)Rearrangement

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
68
Draw arrows for the following reaction. 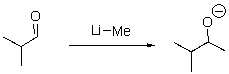


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following is the electrophilic site in the following reaction? 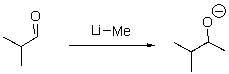
A)C of the carbonyl
B)O
C)Li
D)Me

A)C of the carbonyl
B)O
C)Li
D)Me

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
70
Identify the nucleophilic and electrophilic sites in the following reaction. 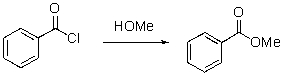


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
71
What pattern is the fourth step of this reaction? 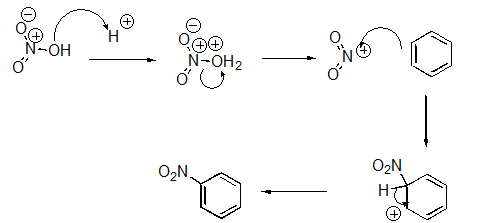
A)Proton Transfer
B)Loss of Leaving Group
C)Nucleophilic Attack
D)Rearrangement

A)Proton Transfer
B)Loss of Leaving Group
C)Nucleophilic Attack
D)Rearrangement

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
72
What pattern is the second step of this reaction? 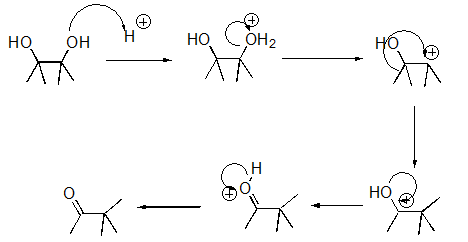
A)Proton Transfer
B)Loss of Leaving Group
C)Nucleophilic Attack
D)Rearrangement

A)Proton Transfer
B)Loss of Leaving Group
C)Nucleophilic Attack
D)Rearrangement

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
73
Will the following cation undergo rearrangement? 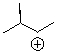


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
74
Identify the sequence of steps in the following reaction. 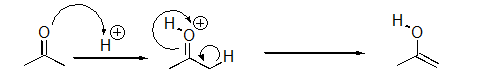
A)Proton transfer,Proton transfer
B)Proton transfer,Loss of leaving group
C)Nucleophilic attack,Proton transfer
D)Proton transfer,Nucleophilic attack
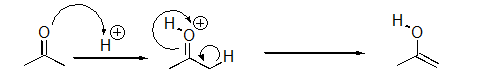
A)Proton transfer,Proton transfer
B)Proton transfer,Loss of leaving group
C)Nucleophilic attack,Proton transfer
D)Proton transfer,Nucleophilic attack

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
75
What pattern is the second step of this reaction? 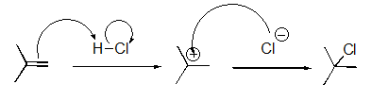
A)Proton Transfer
B)Loss of Leaving Group
C)Nucleophilic Attack
D)Rearrangement

A)Proton Transfer
B)Loss of Leaving Group
C)Nucleophilic Attack
D)Rearrangement

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following is the electrophilic site in the following reaction? 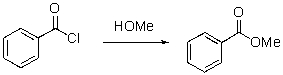
A)C of carbonyl
B)O of carbonyl
C)Cl
D)O of alcohol

A)C of carbonyl
B)O of carbonyl
C)Cl
D)O of alcohol

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
77
Draw arrows for each step of the following reaction. 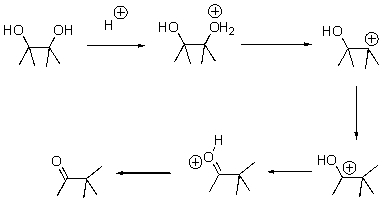


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
78
Identify the sequence of steps in the following reaction. 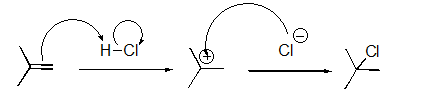
A)Proton transfer,Proton transfer
B)Proton transfer,Loss of leaving group
C)Nucleophilic attack,Proton transfer
D)Proton transfer,Nucleophilic attack

A)Proton transfer,Proton transfer
B)Proton transfer,Loss of leaving group
C)Nucleophilic attack,Proton transfer
D)Proton transfer,Nucleophilic attack

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
79
What pattern is the second step of this reaction? 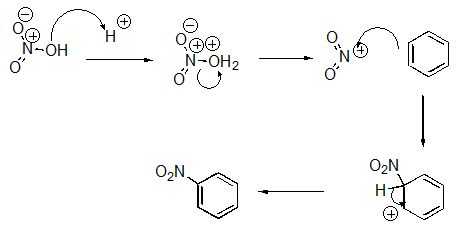
A)Proton Transfer
B)Loss of Leaving Group
C)Nucleophilic Attack
D)Rearrangement

A)Proton Transfer
B)Loss of Leaving Group
C)Nucleophilic Attack
D)Rearrangement

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
80
Draw arrows for each step of the following reaction. 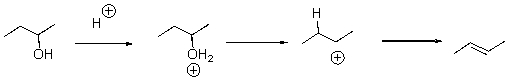


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck



