Deck 12: Structure Determination: Mass Spectrometry and Infrared Spectroscopy
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/42
Play
Full screen (f)
Deck 12: Structure Determination: Mass Spectrometry and Infrared Spectroscopy
1
Exhibit 12-2
Use the data below to answer the following question(s).
Loratidine is the active ingredient in the antihistamine Claritin .Mass spectral analysis of loratidine shows M+ at m/z = 382 and M+ at m/z = 384 in an approximate ratio of 3:1 in intensity.
Refer to Exhibit 12-2.Loratidine is known to contain nitrogen.What is the minimum number of nitrogens in loratidine?
Use the data below to answer the following question(s).
Loratidine is the active ingredient in the antihistamine Claritin .Mass spectral analysis of loratidine shows M+ at m/z = 382 and M+ at m/z = 384 in an approximate ratio of 3:1 in intensity.
Refer to Exhibit 12-2.Loratidine is known to contain nitrogen.What is the minimum number of nitrogens in loratidine?
Since the molecular ions are even numbers,loratidine must have an even number of nitrogen atoms.Therefore,the minimum number of nitrogen atoms in loratidine is two.
2
Exhibit 12-3
For each compound below,what are the masses of the charged fragments produced by the indicated cleavage pathways?
Refer to Exhibit 12-3.Alpha cleavage of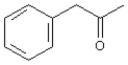
For each compound below,what are the masses of the charged fragments produced by the indicated cleavage pathways?
Refer to Exhibit 12-3.Alpha cleavage of

This compound,phenylacetone,has the formula C9H10O and a nominal mass of 134.Alpha cleavage will result in charged fragments at m/z = 43 and m/z = 119. 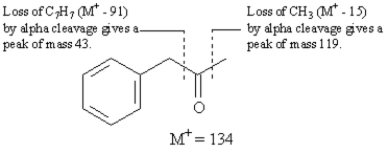

3
Exhibit 12-3
For each compound below,what are the masses of the charged fragments produced by the indicated cleavage pathways?
Refer to Exhibit 12-3.Alpha cleavage and dehydration of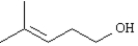
For each compound below,what are the masses of the charged fragments produced by the indicated cleavage pathways?
Refer to Exhibit 12-3.Alpha cleavage and dehydration of

This compound,4-methyl-3-penten-1-ol,has the formula C6H12O and a nominal mass of 100.Alpha cleavage will result in a charged fragment at m/z = 31.Dehydration (M+ - 18) will result in a charged fragment at m/z = 82. 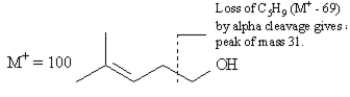

4
Exhibit 12-2
Use the data below to answer the following question(s).
Loratidine is the active ingredient in the antihistamine Claritin .Mass spectral analysis of loratidine shows M+ at m/z = 382 and M+ at m/z = 384 in an approximate ratio of 3:1 in intensity.
Refer to Exhibit 12-2.The mass spectral data indicates that loratidine contains:
A)fluorine
B)chlorine
C)bromine
D)iodine
Use the data below to answer the following question(s).
Loratidine is the active ingredient in the antihistamine Claritin .Mass spectral analysis of loratidine shows M+ at m/z = 382 and M+ at m/z = 384 in an approximate ratio of 3:1 in intensity.
Refer to Exhibit 12-2.The mass spectral data indicates that loratidine contains:
A)fluorine
B)chlorine
C)bromine
D)iodine

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
5
Exhibit 12-5
Refer to the mass spectrum of 2-methylbutane shown below to answer the following question(s).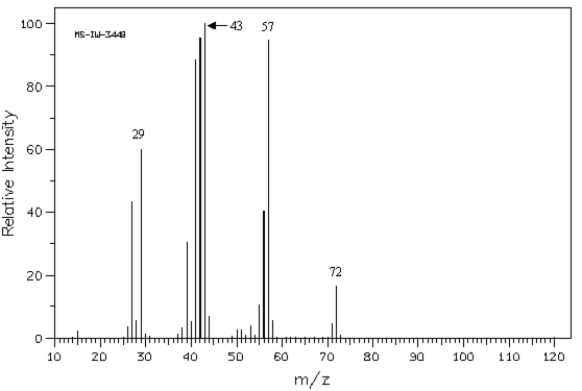 Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Refer to Exhibit 12-5.What peak represents M+?
Refer to the mass spectrum of 2-methylbutane shown below to answer the following question(s).
 Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/Refer to Exhibit 12-5.What peak represents M+?

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
6
The amount of energy in electromagnetic radiation is related to the frequency and wavelength of the radiation.High energy radiation like gamma rays is of:
A)low frequency and short wavelength
B)low frequency and long wavelength
C)high frequency and short wavelength
D)high frequency and long wavelength
A)low frequency and short wavelength
B)low frequency and long wavelength
C)high frequency and short wavelength
D)high frequency and long wavelength

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
7
The amount of energy in infrared light corresponds to:
A)the amount of energy needed to promote one electron from a bonding to an antibonding molecular orbital
B)the amount of energy needed to "flip" the spin of a 13C or 1H nucleus
C)the amount of energy needed to strip a molecule of one electron to generate a cation radical
D)the amount of energy needed to increase certain molecular motions,such as bond vibrations,in organic molecules
A)the amount of energy needed to promote one electron from a bonding to an antibonding molecular orbital
B)the amount of energy needed to "flip" the spin of a 13C or 1H nucleus
C)the amount of energy needed to strip a molecule of one electron to generate a cation radical
D)the amount of energy needed to increase certain molecular motions,such as bond vibrations,in organic molecules

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following statements best describes the base peak in a mass spectrum?
A)The peak from the most stable radical.
B)The peak from the species that has the isotope with the highest atomic number.
C)The peak of highest intensity.
D)The peak from the molecule minus an electron.
A)The peak from the most stable radical.
B)The peak from the species that has the isotope with the highest atomic number.
C)The peak of highest intensity.
D)The peak from the molecule minus an electron.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
9
The sugar glucose contains C,H,and O and has a mass of 180.0634 amu as determined by high-resolution mass spectrometry.Glucose contains an equal number of carbon and oxygen atoms.What is the molecular formula of glucose? (1H = 1.00783 amu,12C = 12.00000 amu,16O = 15.99491 amu)

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
10
Exhibit 12-4
The following question(s) refer to the mass spectrum shown below.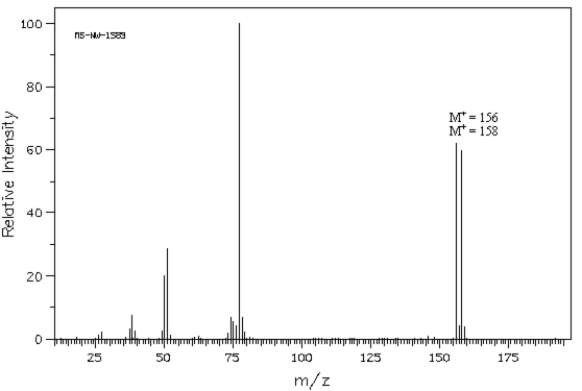 Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Refer to Exhibit 12-4.Calculate a possible molecular formula for this compound.
The following question(s) refer to the mass spectrum shown below.
 Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/Refer to Exhibit 12-4.Calculate a possible molecular formula for this compound.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
11
Exhibit 12-5
Refer to the mass spectrum of 2-methylbutane shown below to answer the following question(s). Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Refer to Exhibit 12-5.What peak represents the base peak?
Refer to the mass spectrum of 2-methylbutane shown below to answer the following question(s).
 Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/Refer to Exhibit 12-5.What peak represents the base peak?

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
12
Both mass spectrometry and infrared spectroscopy involve the interaction of molecules with electromagnetic energy?

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
13
Exhibit 12-1
Select the most reasonable formula for the compounds with the following mass spectral data.
Refer to Exhibit 12-1.M+ m/z = 136 and M+ at m/z = 138 of approximately equal intensity
A)C6H13OCl
B)C4H9Br
C)C10H16
D)C9H12O
Select the most reasonable formula for the compounds with the following mass spectral data.
Refer to Exhibit 12-1.M+ m/z = 136 and M+ at m/z = 138 of approximately equal intensity
A)C6H13OCl
B)C4H9Br
C)C10H16
D)C9H12O

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
14
Could you distinguish 2,2-dimethylbutane from 2-methylpentane using mass spectrometry?

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
15
Exhibit 12-4
The following question(s) refer to the mass spectrum shown below. Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Refer to Exhibit 12-4.This compound contains C,H,and one other atom.Identify the other atom from the mass spectrum and explain your reasoning.
The following question(s) refer to the mass spectrum shown below.
 Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/Refer to Exhibit 12-4.This compound contains C,H,and one other atom.Identify the other atom from the mass spectrum and explain your reasoning.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
16
Below is the mass spectrum of an unknown hydrocarbon.In addition,this hydrocarbon shows characteristic absorption at 2100 cm−1 in its IR spectrum.Give the structure of this unknown. 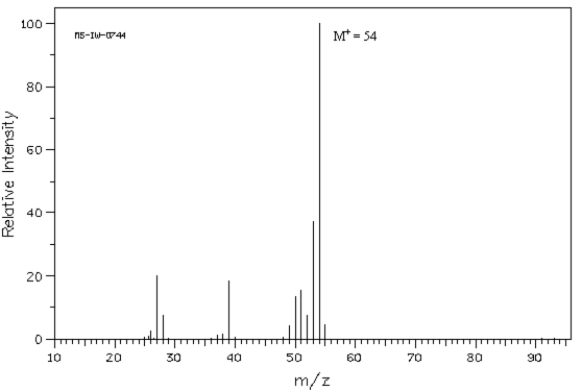 Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
 Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
17
Exhibit 12-1
Select the most reasonable formula for the compounds with the following mass spectral data.
Refer to Exhibit 12-1.M+ at m/z = 101 with a minor M+1 peak
A)C5H6Cl
B)C5H12N2
C)C6H15N
D)C9H12O
Select the most reasonable formula for the compounds with the following mass spectral data.
Refer to Exhibit 12-1.M+ at m/z = 101 with a minor M+1 peak
A)C5H6Cl
B)C5H12N2
C)C6H15N
D)C9H12O

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
18
Exhibit 12-5
Refer to the mass spectrum of 2-methylbutane shown below to answer the following question(s). Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Refer to Exhibit 12-5.Propose structures for fragment ions at m/z = 57,43,and 29.
Refer to the mass spectrum of 2-methylbutane shown below to answer the following question(s).
 Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/Refer to Exhibit 12-5.Propose structures for fragment ions at m/z = 57,43,and 29.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
19
Exhibit 12-4
The following question(s) refer to the mass spectrum shown below. Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Refer to Exhibit 12-4.Propose a structure for this compound.
The following question(s) refer to the mass spectrum shown below.
 Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/Refer to Exhibit 12-4.Propose a structure for this compound.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
20
Exhibit 12-5
Refer to the mass spectrum of 2-methylbutane shown below to answer the following question(s). Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Refer to Exhibit 12-5.Which peak represents the parent peak?
Refer to the mass spectrum of 2-methylbutane shown below to answer the following question(s).
 Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/Refer to Exhibit 12-5.Which peak represents the parent peak?

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
21
Consider the following mass spectrum 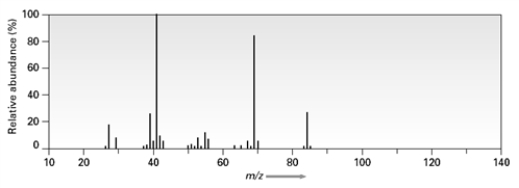 Which of the following compounds might have been used to produce this spectrum?
Which of the following compounds might have been used to produce this spectrum?
A)C6H14
B)C6H12
C)C6H10
D)C5H12O
 Which of the following compounds might have been used to produce this spectrum?
Which of the following compounds might have been used to produce this spectrum?A)C6H14
B)C6H12
C)C6H10
D)C5H12O

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
22
Examining the infrared spectrum of a compound allows us to:
A)determine the types of functional groups present in the compound
B)determine the carbon-hydrogen framework of the compound
C)determine the molecular weight of the compound
D)determine the nature of the conjugated pi electron system in the compound
A)determine the types of functional groups present in the compound
B)determine the carbon-hydrogen framework of the compound
C)determine the molecular weight of the compound
D)determine the nature of the conjugated pi electron system in the compound

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
23
When the molecular ion of 2,2-dimethylpentane fragments,the positive charge is most likely to stay on:
A)the methyl group.
B)the ethyl group.
C)the propyl group.
D)the tert-butyl group.
A)the methyl group.
B)the ethyl group.
C)the propyl group.
D)the tert-butyl group.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
24
Predict the m/z position of the parent peak,the peak due to dehydration of the alcohol and α-cleavage of the carbonyl in the mass spectrum of the compound shown below. 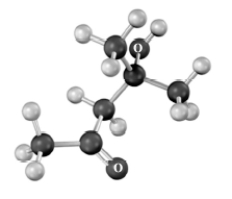


Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
25
Assume you are carrying out the conversion of 1-bromobutane to 1-butanol.How could you use IR spectroscopy to determine when the reaction is complete? 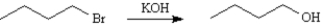
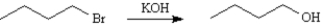

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
26
Exhibit 12-6
MATCH each of the following groups of bond-types to the region of the infrared spectrum in which their absorptions occur.Place the letter of the region in the blank to the left of the bond-type.
_____ N−H,C−H,and O−H stretching and bending motions.
A)4000 to 2500 cm−1
B)2500 to 2000 cm−1
C)2000 to 1500 cm−1
D)below 1500 cm−1
MATCH each of the following groups of bond-types to the region of the infrared spectrum in which their absorptions occur.Place the letter of the region in the blank to the left of the bond-type.
_____ N−H,C−H,and O−H stretching and bending motions.
A)4000 to 2500 cm−1
B)2500 to 2000 cm−1
C)2000 to 1500 cm−1
D)below 1500 cm−1

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
27
What m/z ratio would expect to find in the mass spectrum of the following compound due to dehydration? 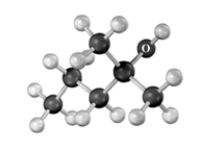
A)102
B)87
C)85
D)84
E)59

A)102
B)87
C)85
D)84
E)59

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
28
When 2-bromopropane reacts with ethoxide ion,two products are formed;one is the product of SN2 substitution and the other is the product of E2 elimination.Write the structures of both products,and tell how they could be distinguished using IR spectroscopy.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
29
Exhibit 12-6
MATCH each of the following groups of bond-types to the region of the infrared spectrum in which their absorptions occur.Place the letter of the region in the blank to the left of the bond-type.
_____ triple bond stretching vibrations.
A)4000 to 2500 cm−1
B)2500 to 2000 cm−1
C)2000 to 1500 cm−1
D)below 1500 cm−1
MATCH each of the following groups of bond-types to the region of the infrared spectrum in which their absorptions occur.Place the letter of the region in the blank to the left of the bond-type.
_____ triple bond stretching vibrations.
A)4000 to 2500 cm−1
B)2500 to 2000 cm−1
C)2000 to 1500 cm−1
D)below 1500 cm−1

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
30
Exhibit 12-6
MATCH each of the following groups of bond-types to the region of the infrared spectrum in which their absorptions occur.Place the letter of the region in the blank to the left of the bond-type.
_____ C=O,C=N,and C=C bond absorptions.
A)4000 to 2500 cm−1
B)2500 to 2000 cm−1
C)2000 to 1500 cm−1
D)below 1500 cm−1
MATCH each of the following groups of bond-types to the region of the infrared spectrum in which their absorptions occur.Place the letter of the region in the blank to the left of the bond-type.
_____ C=O,C=N,and C=C bond absorptions.
A)4000 to 2500 cm−1
B)2500 to 2000 cm−1
C)2000 to 1500 cm−1
D)below 1500 cm−1

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
31
Consider the following spectrum. 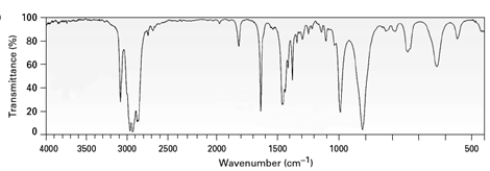 The compound used to generate this spectrum might have which of the following molecular formulas?
The compound used to generate this spectrum might have which of the following molecular formulas?
A)C7H16
B)C7H16O
C)C7H14
D)C7H12
E)either c or d
 The compound used to generate this spectrum might have which of the following molecular formulas?
The compound used to generate this spectrum might have which of the following molecular formulas?A)C7H16
B)C7H16O
C)C7H14
D)C7H12
E)either c or d

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following regions of the IR spectrum would be significantly different for the following two substances? 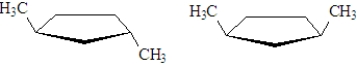
A)4000 - 2850 cm-1
B)2850 - 2000 cm-1
C)2000 - 1500 cm-1
D)1500 - 400 cm-1
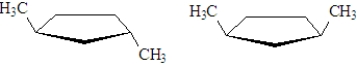
A)4000 - 2850 cm-1
B)2850 - 2000 cm-1
C)2000 - 1500 cm-1
D)1500 - 400 cm-1

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
33
In which of the following regions of the electromagnetic spectrum is the frequency lower than in the IR region?
A)radio wave
B)visible
C)ultraviolet
D)X ray
A)radio wave
B)visible
C)ultraviolet
D)X ray

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
34
Cyclohexene and 2-hexyne both have the molecular formula C6H10.How would you use infrared spectroscopy to distinguish between the two compounds?

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
35
Mass spectrometry and infrared spectroscopy are complementary techniques because:
A)Mass spectrometry provides information about the molar mass and formula while infrared spectroscopy helps identify the functional groups in the formula.
B)Infrared spectroscopy provides information about the molar mass and formula while mass spectrometry helps identify the functional groups in the formula.
C)Mass spectrometry provides information about the carbon-hydrogen framework while infrared spectroscopy helps identify the functional groups in the framework.
D)Infrared spectroscopy provides information about the carbon-hydrogen framework while mass spectrometry helps identify the functional groups in the framework.
A)Mass spectrometry provides information about the molar mass and formula while infrared spectroscopy helps identify the functional groups in the formula.
B)Infrared spectroscopy provides information about the molar mass and formula while mass spectrometry helps identify the functional groups in the formula.
C)Mass spectrometry provides information about the carbon-hydrogen framework while infrared spectroscopy helps identify the functional groups in the framework.
D)Infrared spectroscopy provides information about the carbon-hydrogen framework while mass spectrometry helps identify the functional groups in the framework.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
36
Exhibit 12-6
MATCH each of the following groups of bond-types to the region of the infrared spectrum in which their absorptions occur.Place the letter of the region in the blank to the left of the bond-type.
_____ C−C,C−O,C−N,and C−X single-bond vibrations.
A)4000 to 2500 cm−1
B)2500 to 2000 cm−1
C)2000 to 1500 cm−1
D)below 1500 cm−1
MATCH each of the following groups of bond-types to the region of the infrared spectrum in which their absorptions occur.Place the letter of the region in the blank to the left of the bond-type.
_____ C−C,C−O,C−N,and C−X single-bond vibrations.
A)4000 to 2500 cm−1
B)2500 to 2000 cm−1
C)2000 to 1500 cm−1
D)below 1500 cm−1

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
37
What are the charged fragments produced if 4,5-dimethylhex-1-en-3-ol undergoes alpha cleavage?

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
38
Ethyl acetate and 2-butene-1,4-diol both have the molecular formula C4H8O2.How would you use infrared spectroscopy to distinguish between the two?

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following provides information about the functional groups present in a sample?
A)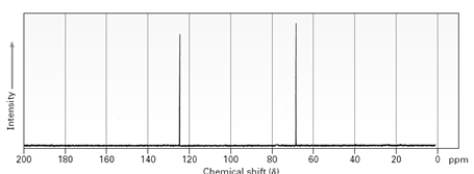
B)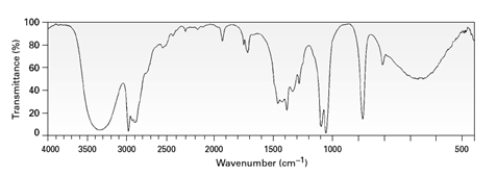
C)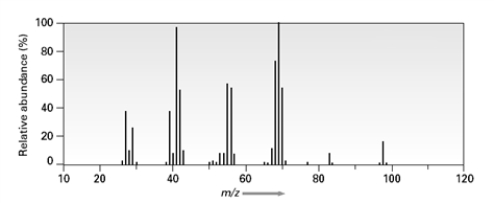
D)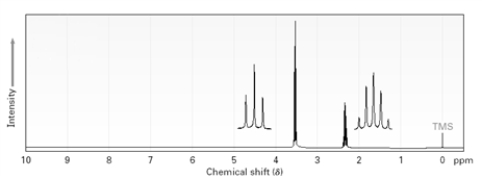
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following regions of the electromagnetic spectrum would have insufficient energy to produce the following energy transition? 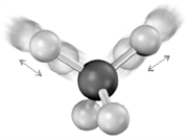
A)radio wave
B)visible
C)ultraviolet
D)X ray

A)radio wave
B)visible
C)ultraviolet
D)X ray

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
41
Both methylcycloheptane and ethylcyclohexane have the same molecular mass.Explain how it is possible to distinguish between the two compounds by studying and comparing both their mass spectra.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
42
The peak in the IR absorption spectra for the alkyne group in acetylene would be observed in the range of:
A)2100-2260 cm-1
B)2370-2650 cm-1
C)2700-2830 cm-1
D)2900-3210 cm-1
A)2100-2260 cm-1
B)2370-2650 cm-1
C)2700-2830 cm-1
D)2900-3210 cm-1

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck



