Deck 7: Alkenes: Structure and Reactivity
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/37
Play
Full screen (f)
Deck 7: Alkenes: Structure and Reactivity
1
Draw: (3E)-3,7-dimethyl-1,3,6-octatriene
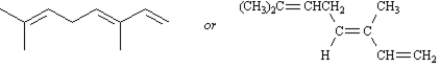
2
Exhibit 7-10
Consider the following reaction:
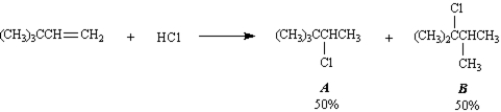
Refer to Exhibit 7-10.Below are all the chemical structures and intermediates involved in this reaction.On the structures provided,show all electron flow using the arrow formalism for the complete stepwise mechanism for this reaction.
Consider the following reaction:

Refer to Exhibit 7-10.Below are all the chemical structures and intermediates involved in this reaction.On the structures provided,show all electron flow using the arrow formalism for the complete stepwise mechanism for this reaction.

3
Draw: 3,6-dimethyl-1,4-cyclohexadiene
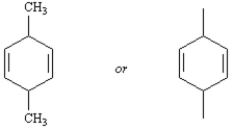
4
Rank the carbocations below in order of increasing stability (least stable = 1;most stable = 3).Place the number corresponding to the carbocation's relative stability in the blank below the structure. 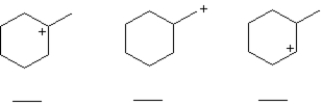


Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
5
Exhibit 7-2
Dieldrin,C12H8Cl6O,is a pentacyclic compound formerly used as an insecticide.
Refer to Exhibit 7-2.How many double bonds does dieldrin have?
Dieldrin,C12H8Cl6O,is a pentacyclic compound formerly used as an insecticide.
Refer to Exhibit 7-2.How many double bonds does dieldrin have?

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
6
The original question was combined with #10.This placeholder question is here to maintain the integrity of the numbering system between the printed copy and ExamView.Therefore,it has been marked "do not use on test" in ExamView's question information dialog.As a result,this placeholder question is automatically prevented from being chosen as a test question.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
7
Write the complete stepwise mechanism for the following reaction.Show all intermediate structures and all electron flow with arrows. 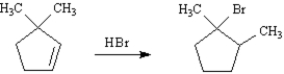


Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
8
Arrange the following bicyclic alkenes in order of increasing stability (least stable to most stable). 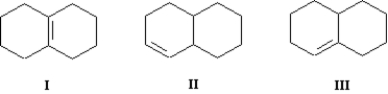
A)III < II < I
B)I < II < III
C)I < III < II
D)II < III < I

A)III < II < I
B)I < II < III
C)I < III < II
D)II < III < I

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
9
Exhibit 7-4
Provide names for each structure below.Be sure to include the cis,trans or E,Z designations where applicable.
Name: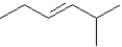
Provide names for each structure below.Be sure to include the cis,trans or E,Z designations where applicable.
Name:


Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
10
Exhibit 7-3
2-Pentene is an example of a disubstituted alkene.
CH3CH=CHCH2CH3
Refer to Exhibit 7-3.
A)Draw the cis and trans isomers of 2-pentene and label them.
B)Circle the isomer which is most stable.
2-Pentene is an example of a disubstituted alkene.
CH3CH=CHCH2CH3
Refer to Exhibit 7-3.
A)Draw the cis and trans isomers of 2-pentene and label them.
B)Circle the isomer which is most stable.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
11
Exhibit 7-9
Consider the following reaction:
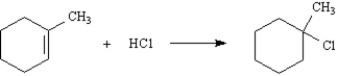
Draw a qualitative reaction energy diagram for this reaction.Label the positions of all reactants,intermediates and products.
Consider the following reaction:
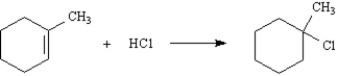
Draw a qualitative reaction energy diagram for this reaction.Label the positions of all reactants,intermediates and products.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
12
Exhibit 7-2
Dieldrin,C12H8Cl6O,is a pentacyclic compound formerly used as an insecticide.
Refer to Exhibit 7-2.Calculate the degree of unsaturation for Dieldrin.Show calculations for credit.
Dieldrin,C12H8Cl6O,is a pentacyclic compound formerly used as an insecticide.
Refer to Exhibit 7-2.Calculate the degree of unsaturation for Dieldrin.Show calculations for credit.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
13
Exhibit 7-4
Provide names for each structure below.Be sure to include the cis,trans or E,Z designations where applicable.
Name: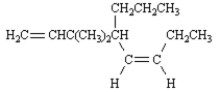
Provide names for each structure below.Be sure to include the cis,trans or E,Z designations where applicable.
Name:


Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
14
Exhibit 7-13
The reaction of isobutene with HBr in ether gives one of the two products below as the major product.Answer the following question(s) about this reaction.
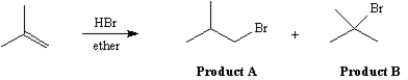
Refer to Exhibit 7-13.Which product would be the major product?
The reaction of isobutene with HBr in ether gives one of the two products below as the major product.Answer the following question(s) about this reaction.

Refer to Exhibit 7-13.Which product would be the major product?

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
15
Draw: cis-2-hexene

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
16
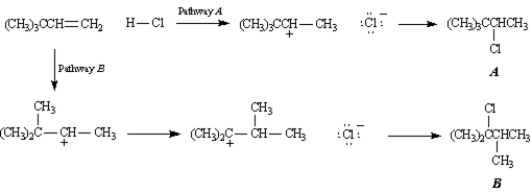
Exhibit 7-10
Consider the following reaction:

Refer to Exhibit 7-10.Why does the rearrangement that results in the formation of product B occur?
Consider the following reaction:

Refer to Exhibit 7-10.Why does the rearrangement that results in the formation of product B occur?

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
17
Draw: trans-4,4-dimethyl-2-pentene

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
18
Exhibit 7-4
Provide names for each structure below.Be sure to include the cis,trans or E,Z designations where applicable.
Name: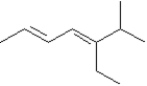
Provide names for each structure below.Be sure to include the cis,trans or E,Z designations where applicable.
Name:


Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
19
Exhibit 7-4
Provide names for each structure below.Be sure to include the cis,trans or E,Z designations where applicable.
Name: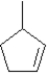
Provide names for each structure below.Be sure to include the cis,trans or E,Z designations where applicable.
Name:


Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
20
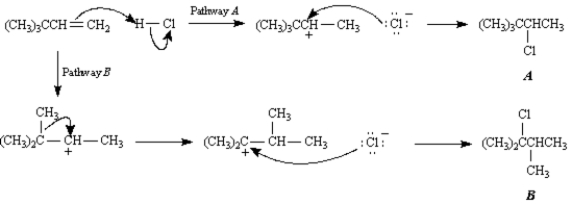
Exhibit 7-9
Consider the following reaction:
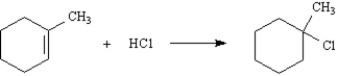
Write the complete stepwise mechanism for this reaction.Show all intermediate structures and show all electron flow using the curved arrow convention.
Consider the following reaction:
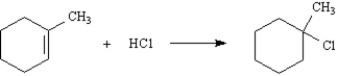
Write the complete stepwise mechanism for this reaction.Show all intermediate structures and show all electron flow using the curved arrow convention.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following does not characterize a more highly substituted carbocation?
A)forms more rapidly than less highly substituted carbocations
B)forms product more rapidly than less highly substituted carbocations
C)has a larger ΔG± than less highly substituted carbocations
D)is more stable than less highly substituted carbocations
E)All of these characterize a highly substituted carbocation.
A)forms more rapidly than less highly substituted carbocations
B)forms product more rapidly than less highly substituted carbocations
C)has a larger ΔG± than less highly substituted carbocations
D)is more stable than less highly substituted carbocations
E)All of these characterize a highly substituted carbocation.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
22
Exhibit 7-13
The reaction of isobutene with HBr in ether gives one of the two products below as the major product.Answer the following question(s) about this reaction.

Refer to Exhibit 7-13.Which product would have a higher energy transition state for the formation of the intermediate leading to it?
The reaction of isobutene with HBr in ether gives one of the two products below as the major product.Answer the following question(s) about this reaction.

Refer to Exhibit 7-13.Which product would have a higher energy transition state for the formation of the intermediate leading to it?

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
23
Exhibit 7-13
The reaction of isobutene with HBr in ether gives one of the two products below as the major product.Answer the following question(s) about this reaction.

Refer to Exhibit 7-13.Which product would be formed via a primary carbocation?
The reaction of isobutene with HBr in ether gives one of the two products below as the major product.Answer the following question(s) about this reaction.

Refer to Exhibit 7-13.Which product would be formed via a primary carbocation?

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
24
Examine the following biological reaction. 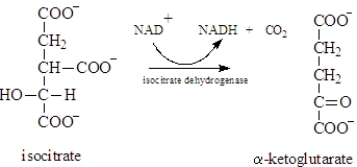 In this reaction,which of the following is not indicated?
In this reaction,which of the following is not indicated?
A)The reaction produces three products.
B)Isocitrate is the primary reactant.
C)Isocitrate dehydrogenase is the catalyst for the reaction,
D)NAD+ is an abbreviation for a required reactant.
E)All indicate the conditions of the reaction.
 In this reaction,which of the following is not indicated?
In this reaction,which of the following is not indicated?A)The reaction produces three products.
B)Isocitrate is the primary reactant.
C)Isocitrate dehydrogenase is the catalyst for the reaction,
D)NAD+ is an abbreviation for a required reactant.
E)All indicate the conditions of the reaction.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
25
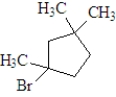
If the following compound was dissolved in ether and treated with HBr,which of the following describes the major product(s) of the reaction? 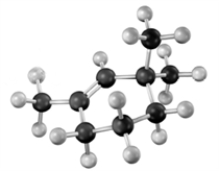
A)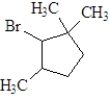
B)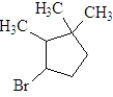
C)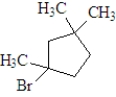
D)a mixture of and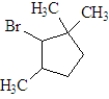

A)

B)

C)

D)a mixture of and



Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
26
Draw the mechanism of the electrophilic addition of HCl to 2-propene.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
27
Draw the mechanism of hydride shift in the reaction between 4-methyl-2-pentene and hydrochloric acid.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
28
Write the reaction mechanism of the electrophilic reaction of HI to 2-pentene.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
29
Examine the following reaction. 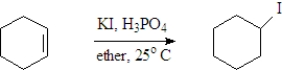 Which of the following is not indicated by this representation?
Which of the following is not indicated by this representation?
A)KI is the solvent
B)Cyclohexene is the reactant of interest.
C)The reaction is carried out at room temperature.
D)The reaction occurs in solution.
E)All indicate the conditions of the reaction.
 Which of the following is not indicated by this representation?
Which of the following is not indicated by this representation?A)KI is the solvent
B)Cyclohexene is the reactant of interest.
C)The reaction is carried out at room temperature.
D)The reaction occurs in solution.
E)All indicate the conditions of the reaction.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
30
Exhibit 7-13
The reaction of isobutene with HBr in ether gives one of the two products below as the major product.Answer the following question(s) about this reaction.

Refer to Exhibit 7-13.According to Hammond's Postulate,which product would have a transition state structure most closely resembling the carbocation intermediate?
The reaction of isobutene with HBr in ether gives one of the two products below as the major product.Answer the following question(s) about this reaction.

Refer to Exhibit 7-13.According to Hammond's Postulate,which product would have a transition state structure most closely resembling the carbocation intermediate?

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
31
The following shows the connectivity of atoms only.Atoms other than carbon and hydrogen are labeled. 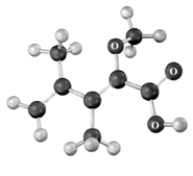 How many degrees of unsaturation are present in the compound?
How many degrees of unsaturation are present in the compound?
A)1
B)2
C)3
D)4
E)5
 How many degrees of unsaturation are present in the compound?
How many degrees of unsaturation are present in the compound?A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
32
Cyclohexanol (-OH bonded to cyclohexane) produces cyclohexene when heated to 70 °C in the presence of a sulfuric acid catalyst.Write the equation for the reaction showing only the reactant of interest and the conditions of the reaction

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
33
Identify the isoprene units in limonene,an essential oil found in oil of lemon and orange. 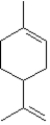


Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
34
If the following compound were dissolved in ether and treated with HCl,which of the following describes the product(s) of the reaction? 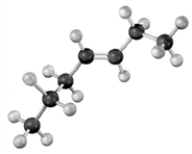
A)3-bromoheptane
B)4-bromoheptane
C)a mixture of 3-bromoheptane and 4-bromoheptane

A)3-bromoheptane
B)4-bromoheptane
C)a mixture of 3-bromoheptane and 4-bromoheptane

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
35
Exhibit 7-13
The reaction of isobutene with HBr in ether gives one of the two products below as the major product.Answer the following question(s) about this reaction.

Refer to Exhibit 7-13.Which product would be formed by a carbocation experiencing the greatest degree of hyperconjugative stabilization?
The reaction of isobutene with HBr in ether gives one of the two products below as the major product.Answer the following question(s) about this reaction.

Refer to Exhibit 7-13.Which product would be formed by a carbocation experiencing the greatest degree of hyperconjugative stabilization?

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
36
Exhibit 7-13
The reaction of isobutene with HBr in ether gives one of the two products below as the major product.Answer the following question(s) about this reaction.

Refer to Exhibit 7-13.Increasing the temperature at which the reaction is run would lead to an increase in the percentage of which product relative to the other product?
The reaction of isobutene with HBr in ether gives one of the two products below as the major product.Answer the following question(s) about this reaction.

Refer to Exhibit 7-13.Increasing the temperature at which the reaction is run would lead to an increase in the percentage of which product relative to the other product?

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
37
Consider the following molecular model. 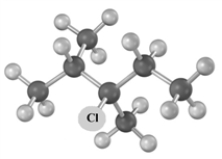 I Which of the following could be used to synthesize this compound?
I Which of the following could be used to synthesize this compound?
A)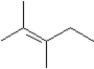
B)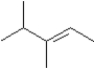
C)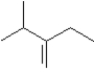
D)Either or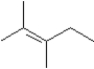
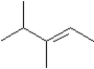
E)Any of the above
 I Which of the following could be used to synthesize this compound?
I Which of the following could be used to synthesize this compound?A)

B)

C)

D)Either or


E)Any of the above

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck



