Deck 5: Stereochemistry at Tetrahedral Centers
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/43
Play
Full screen (f)
Deck 5: Stereochemistry at Tetrahedral Centers
1
Exhibit 5-3
Assign R,S configurations to each indicated chirality center in the molecules below.
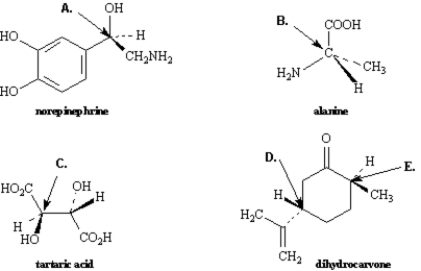
Refer to Exhibit 5-3.The configuration of this carbon atom (A) is _____.
Assign R,S configurations to each indicated chirality center in the molecules below.

Refer to Exhibit 5-3.The configuration of this carbon atom (A) is _____.
R
2
_____ is the reason for "handedness" in molecules;the property of an object that causes it to be nonsuperimposable on its mirror image.
A)racemates
B)chirality center
C)chirality
D)diastereomers
E)enantiomers
F)meso compounds
G)optically active
H)prochirality center
I)optically inactive
J)achiral
A)racemates
B)chirality center
C)chirality
D)diastereomers
E)enantiomers
F)meso compounds
G)optically active
H)prochirality center
I)optically inactive
J)achiral
chirality
3
Exhibit 5-5
Label each pair of stereoisomers below as:
_____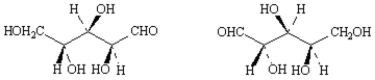
A)enantiomers
B)diastereomers
C)identical
Place the letter of the correct answer in the blank to the left of the pair of stereoisomers.
Label each pair of stereoisomers below as:
_____
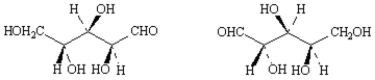
A)enantiomers
B)diastereomers
C)identical
Place the letter of the correct answer in the blank to the left of the pair of stereoisomers.
diastereomers
4
Exhibit 5-5
Label each pair of stereoisomers below as:
_____
A)enantiomers
B)diastereomers
C)identical
Place the letter of the correct answer in the blank to the left of the pair of stereoisomers.
Label each pair of stereoisomers below as:
_____

A)enantiomers
B)diastereomers
C)identical
Place the letter of the correct answer in the blank to the left of the pair of stereoisomers.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
5
Exhibit 5-3
Assign R,S configurations to each indicated chirality center in the molecules below.

Refer to Exhibit 5-3.The configuration of this carbon atom (B) is _____.
Assign R,S configurations to each indicated chirality center in the molecules below.

Refer to Exhibit 5-3.The configuration of this carbon atom (B) is _____.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
6
_____ is an atom in a molecule that is bonded to four different atoms or groups of atoms.
A)racemates
B)chirality center
C)chirality
D)diastereomers
E)enantiomers
F)meso compounds
G)optically active
H)prochirality center
I)optically inactive
J)achiral
A)racemates
B)chirality center
C)chirality
D)diastereomers
E)enantiomers
F)meso compounds
G)optically active
H)prochirality center
I)optically inactive
J)achiral

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
7
Exhibit 5-4
Consider the structure of streptimidone to answer the following question(s).
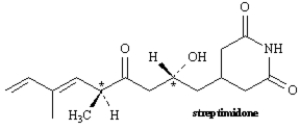
Refer to Exhibit 5-4.Will streptimidone have a meso stereoisomer? Explain your answer.
Consider the structure of streptimidone to answer the following question(s).

Refer to Exhibit 5-4.Will streptimidone have a meso stereoisomer? Explain your answer.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
8
The specific rotation of a compound is denoted by the symbol:
A)R
B)S
C)α
D)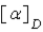
A)R
B)S
C)α
D)
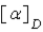

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
9
_____ are stereoisomers that are not mirror images.
A)racemates
B)chirality center
C)chirality
D)diastereomers
E)enantiomers
F)meso compounds
G)optically active
H)prochirality center
I)optically inactive
J)achiral
A)racemates
B)chirality center
C)chirality
D)diastereomers
E)enantiomers
F)meso compounds
G)optically active
H)prochirality center
I)optically inactive
J)achiral

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
10
Exhibit 5-3
Assign R,S configurations to each indicated chirality center in the molecules below.

Refer to Exhibit 5-3.The configuration of this carbon atom (D) is _____.
Assign R,S configurations to each indicated chirality center in the molecules below.

Refer to Exhibit 5-3.The configuration of this carbon atom (D) is _____.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
11
Exhibit 5-3
Assign R,S configurations to each indicated chirality center in the molecules below.

Refer to Exhibit 5-3.The configuration of this carbon atom (E) is _____.
Assign R,S configurations to each indicated chirality center in the molecules below.

Refer to Exhibit 5-3.The configuration of this carbon atom (E) is _____.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
12
Exhibit 5-3
Assign R,S configurations to each indicated chirality center in the molecules below.

Refer to Exhibit 5-3.The configuration of this carbon atom (C) is _____.
Assign R,S configurations to each indicated chirality center in the molecules below.

Refer to Exhibit 5-3.The configuration of this carbon atom (C) is _____.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
13
Exhibit 5-4
Consider the structure of streptimidone to answer the following question(s).

Refer to Exhibit 5-4.Based on the number of chirality centers,how many stereoisomers of streptimidone are possible?
Consider the structure of streptimidone to answer the following question(s).

Refer to Exhibit 5-4.Based on the number of chirality centers,how many stereoisomers of streptimidone are possible?

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
14
_____ is an sp3-hybridized atom that can become a chirality center by changing one of its attached groups.
A)racemates
B)chirality center
C)chirality
D)diastereomers
E)enantiomers
F)meso compounds
G)optically active
H)prochirality center
I)optically inactive
J)achiral
A)racemates
B)chirality center
C)chirality
D)diastereomers
E)enantiomers
F)meso compounds
G)optically active
H)prochirality center
I)optically inactive
J)achiral

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
15
_____ are molecules which contain a plane of symmetry and chirality centers,but are achiral.
A)racemates
B)chirality center
C)chirality
D)diastereomers
E)enantiomers
F)meso compounds
G)optically active
H)prochirality center
I)optically inactive
J)achiral
A)racemates
B)chirality center
C)chirality
D)diastereomers
E)enantiomers
F)meso compounds
G)optically active
H)prochirality center
I)optically inactive
J)achiral

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
16
____ are organic molecules which rotate the plane of polarization of plane-polarized light.
A)racemates
B)chirality center
C)chirality
D)diastereomers
E)enantiomers
F)meso compounds
G)optically active
H)prochirality center
I)optically inactive
J)achiral
A)racemates
B)chirality center
C)chirality
D)diastereomers
E)enantiomers
F)meso compounds
G)optically active
H)prochirality center
I)optically inactive
J)achiral

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
17
Exhibit 5-5
Label each pair of stereoisomers below as:
_____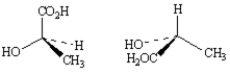
A)enantiomers
B)diastereomers
C)identical
Place the letter of the correct answer in the blank to the left of the pair of stereoisomers.
Label each pair of stereoisomers below as:
_____

A)enantiomers
B)diastereomers
C)identical
Place the letter of the correct answer in the blank to the left of the pair of stereoisomers.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
18
Exhibit 5-4
Consider the structure of streptimidone to answer the following question(s).

Refer to Exhibit 5-4.Assign R or S configuration to each chirality center indicated in streptimidone.
Consider the structure of streptimidone to answer the following question(s).

Refer to Exhibit 5-4.Assign R or S configuration to each chirality center indicated in streptimidone.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
19
Exhibit 5-5
Label each pair of stereoisomers below as:
_____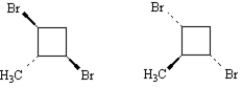
A)enantiomers
B)diastereomers
C)identical
Place the letter of the correct answer in the blank to the left of the pair of stereoisomers.
Label each pair of stereoisomers below as:
_____
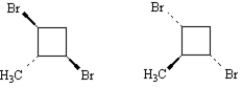
A)enantiomers
B)diastereomers
C)identical
Place the letter of the correct answer in the blank to the left of the pair of stereoisomers.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
20
Exhibit 5-5
Label each pair of stereoisomers below as:
_____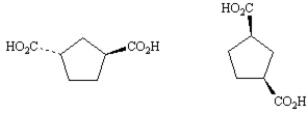
A)enantiomers
B)diastereomers
C)identical
Place the letter of the correct answer in the blank to the left of the pair of stereoisomers.
Label each pair of stereoisomers below as:
_____

A)enantiomers
B)diastereomers
C)identical
Place the letter of the correct answer in the blank to the left of the pair of stereoisomers.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
21
(2R,3S)-Dibromobutane is:
A)optically active.
B)racemic.
C)dextrorotatory.
D)a meso compound.
A)optically active.
B)racemic.
C)dextrorotatory.
D)a meso compound.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
22
Exhibit 5-6
Refer to the structure below to answer the following question(s).
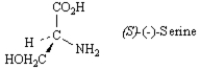
Refer to Exhibit 5-6.Draw the enantiomer of (S)-(−)-serine in a wedge-dash projection.
Refer to the structure below to answer the following question(s).

Refer to Exhibit 5-6.Draw the enantiomer of (S)-(−)-serine in a wedge-dash projection.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
23
Exhibit 5-5
Label each pair of stereoisomers below as:
_____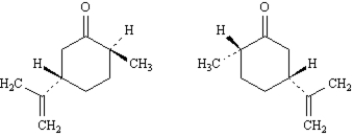
A)enantiomers
B)diastereomers
C)identical
Place the letter of the correct answer in the blank to the left of the pair of stereoisomers.
Label each pair of stereoisomers below as:
_____

A)enantiomers
B)diastereomers
C)identical
Place the letter of the correct answer in the blank to the left of the pair of stereoisomers.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
24
Exhibit 5-7
A natural product having [α]D = +40.3° has been isolated and purified.
Refer to Exhibit 5-7.Two structures have been proposed for this natural product.Circle the structure that is consistent with the information presented and briefly explain your choice.
![Exhibit 5-7 A natural product having [α]<sub>D</sub> = +40.3° has been isolated and purified. Refer to Exhibit 5-7.Two structures have been proposed for this natural product.Circle the structure that is consistent with the information presented and briefly explain your choice. ](https://d2lvgg3v3hfg70.cloudfront.net/TB4944/11eab917_151f_2441_99e6_dd784b5480ad_TB4944_00.jpg)
A natural product having [α]D = +40.3° has been isolated and purified.
Refer to Exhibit 5-7.Two structures have been proposed for this natural product.Circle the structure that is consistent with the information presented and briefly explain your choice.
![Exhibit 5-7 A natural product having [α]<sub>D</sub> = +40.3° has been isolated and purified. Refer to Exhibit 5-7.Two structures have been proposed for this natural product.Circle the structure that is consistent with the information presented and briefly explain your choice. ](https://d2lvgg3v3hfg70.cloudfront.net/TB4944/11eab917_151f_2441_99e6_dd784b5480ad_TB4944_00.jpg)

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
25
Explain why a work glove is chiral but disposable surgical gloves are not.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
26
Exhibit 5-7
A natural product having [α]D = +40.3° has been isolated and purified.
Refer to Exhibit 5-7.This information indicates that the natural product:
A)is racemic.
B)does not rotate plane-polarized light.
C)is levorotatory.
D)is dextrorotatory.
A natural product having [α]D = +40.3° has been isolated and purified.
Refer to Exhibit 5-7.This information indicates that the natural product:
A)is racemic.
B)does not rotate plane-polarized light.
C)is levorotatory.
D)is dextrorotatory.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
27
Draw a wedge-dash projection of (2R,3S)-dibromobutane.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
28
Draw a Newman projection of the most stable conformation of (2R,3S)-dibromobutane sighting down the C2−C3 bond.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
29
Exhibit 5-6
Refer to the structure below to answer the following question(s).

Refer to Exhibit 5-6.Give the complete name of the enantiomer of (S)-(−)-serine.
Refer to the structure below to answer the following question(s).

Refer to Exhibit 5-6.Give the complete name of the enantiomer of (S)-(−)-serine.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following compounds will rotate the plane of polarized light?
A)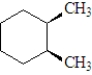
B)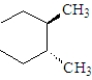
C)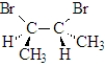
D)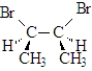
A)

B)

C)
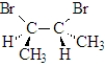
D)


Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following objects is not chiral?
A)baseball glove
B)ballerina slipper
C)corkscrew
D)comb
E)All are chiral.
A)baseball glove
B)ballerina slipper
C)corkscrew
D)comb
E)All are chiral.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
32
Exhibit 5-6
Refer to the structure below to answer the following question(s).

Refer to Exhibit 5-6.(S)-(−)-Serine:
A)is dextrorotatory
B)rotates plane-polarized light in a counterclockwise direction
C)rotates plane-polarized light in a clockwise direction
D)is racemic
Refer to the structure below to answer the following question(s).

Refer to Exhibit 5-6.(S)-(−)-Serine:
A)is dextrorotatory
B)rotates plane-polarized light in a counterclockwise direction
C)rotates plane-polarized light in a clockwise direction
D)is racemic

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
33
In which of the following groups can the carbon atom be a chiral center?
A)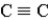
B)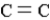
C)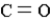
D)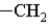
E)The carbon atoms cannot be a chiral center in any of these groups.
A)
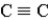
B)
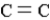
C)
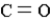
D)
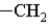
E)The carbon atoms cannot be a chiral center in any of these groups.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
34
Acetoacetate synthase catalyzes the addition of pyruvate to β-ketobutyrate to yield α-aceto-α-hydroxybutyrate.If the addition occurs from the si face of β-ketobutyrate,what is the stereochemistry of the product? 


Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
35
A white solid known to be enantiomerically pure is dissolved in water.This solution produced an observed optical rotation of +45.6°.The solution was allowed to stand and the optical rotation was measured repeatedly producing the data shown in the table.
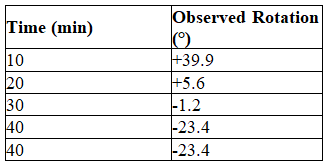
Explain the pattern of observed rotation.

Explain the pattern of observed rotation.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
36
Draw a structure for a singly substituted chiral chloroalkane that contains four carbon atoms.Indicate the chirality center with an asterisk (*).

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
37
Exhibit 5-5
Label each pair of stereoisomers below as:
_____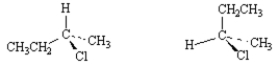
A)enantiomers
B)diastereomers
C)identical
Place the letter of the correct answer in the blank to the left of the pair of stereoisomers.
Label each pair of stereoisomers below as:
_____
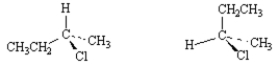
A)enantiomers
B)diastereomers
C)identical
Place the letter of the correct answer in the blank to the left of the pair of stereoisomers.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following would be has the highest priority according to the sequence rules?
A)-SH
B)-NH2
C)-CH3
D)-OH
A)-SH
B)-NH2
C)-CH3
D)-OH

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
39
Estriol,a potent estrogenic hormone,has been isolated from the urine of pregnant women.When 40 mg of estriol is dissolved in 1.0 mL of dioxane and placed in a sample tube with 1 dm path length a rotation of +2.32° is observed.Calculate the specific rotation for estriol.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following arranges the groups in order of decreasing priority according to the sequence rules?
A)CH3 > CH2CH3 > CH2OH > CO2H
B)CH3 > CH2CH3 > CO2H >CH2OH
C)CH2OH > CO2H > CH2CH3 > CH3
D)CO2H > CH2OH > CH2CH3 > CH3
A)CH3 > CH2CH3 > CH2OH > CO2H
B)CH3 > CH2CH3 > CO2H >CH2OH
C)CH2OH > CO2H > CH2CH3 > CH3
D)CO2H > CH2OH > CH2CH3 > CH3

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
41
A compound with specific rotation,[α]D = +35.4° reacts to form a single product.The product of the reaction shows no optical activity.This could mean that the product:
A)is a racemate.
B)does not have any chiral centers.
C)is a meso compound.
D)Any of the above are possible explanations.
A)is a racemate.
B)does not have any chiral centers.
C)is a meso compound.
D)Any of the above are possible explanations.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
42
For which of the following generic substances is it not possible to isolate the enantiomers? (R1,R2 and R3 represent different groups. )
A)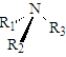
B)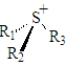
C)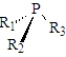
D)It is possible to isolate the enantiomers of all of these.
E)It is not possible to isolate the enantiomers of any of these.
A)
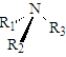
B)
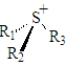
C)
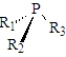
D)It is possible to isolate the enantiomers of all of these.
E)It is not possible to isolate the enantiomers of any of these.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
43
In muscles during strenuous exercise,under anaerobic conditions lactic acid builds up due to the following reaction. 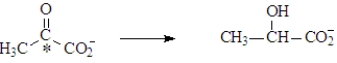 The carbon atom indicated by the asterisk is
The carbon atom indicated by the asterisk is
A)chiral
B)achiral
C)prochiral
D)both achiral and prochiral
 The carbon atom indicated by the asterisk is
The carbon atom indicated by the asterisk isA)chiral
B)achiral
C)prochiral
D)both achiral and prochiral

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck



