In the presence of a small amount of bromine, the following light-promoted reaction has been observed. Write a mechanism for this reaction, making sure to explain how both products are formed. 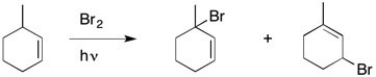
Correct Answer:
Verified
Q14: In the first propagation step of the
Q15: Provide the two propagation steps in the
Q16: Draw the important resonance form/s of the
Q17: The monochlorination of butane with chlorine gas
Q18: Species with unpaired electrons are called _.
Q20: For the compound below, the number of
Q21: For the reaction A + B →
Q22: Given a ΔG° of -8.0 kJ/mol at
Q23: Given a K of 0.45 at 25°C,
Q24: Given a K of 2.2 at 25°C,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents