Consider the reaction of A being converted into B at 25°C. If the ΔG° of this reaction is 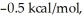 the Keq is ________ and the % conversion is ________.
the Keq is ________ and the % conversion is ________.
A) 0.18; 15%
B) 0.43; 30%
C) 1.0; 50%
D) 2.3; 70%
E) 5.4; 84%
Correct Answer:
Verified
Q35: If the equilibrium constant (Keq) of a
Q36: Given the pKa values for the two
Q37: Consider the reaction of A being converted
Q38: In an exothermic reaction, are stronger bonds
Q39: Which of the following correctly expresses the
Q41: Predict the enthalpy (ΔH) value for the
Q42: Which of the presented mechanisms would be
Q43: Does one expect ΔS° in a propagation
Q44: If stronger bonds are formed and weaker
Q45: Consider the elementary step in the solvolysis
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents