Which of the following statements correctly describes the contribution of 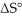 to
to 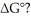
A) The entropy term makes a greater contribution to ΔG° at low temperatures.
B) The entropy term makes a greater contribution to ΔG° at high temperatures.
C) The entropy term makes a greater contribution to ΔG° in exothermic reactions.
D) The entropy term makes a greater contribution to ΔG° in endothermic reactions.
E) The entropy term always makes a more significant contribution to ΔG° than does the enthalpy term.
Correct Answer:
Verified
Q26: If the percentage of the axial onformer
Q27: Consider the transformation of A to B
Q28: If a reaction is exothermic, then _.
A)
Q29: Which of the following is true for
Q30: Given a reaction in which reactant A
Q32: Assume the reaction A + B →
Q33: Consider the reaction of A being converted
Q34: Consider the following substitution reaction with a
Q35: If the equilibrium constant (Keq) of a
Q36: Given the pKa values for the two
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents