Which of the presented mechanisms would be the most energetically favorable and thus the most likely mechanism to actually occur for the following free radical chain reaction?
(bond dissociation energies - H-H = 104 kcal/mol; Cl-Cl = 58 kcal/mol; H-Cl = 103 kcal/mol)
H2 + Cl2 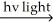 2 HCl
2 HCl
A) H2 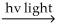 H∙ + H∙
H∙ + H∙
H∙ + Cl2 → Cl∙ + HCl
H∙ + Cl∙ → HCl
B) Cl2 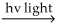 Cl∙ + Cl∙
Cl∙ + Cl∙
Cl∙ + H2 → H∙ + HCl
H∙ + Cl2 → HCl + Cl∙
C) H2 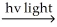 H∙ + H∙
H∙ + H∙
H∙ + Cl2 → Cl∙ + HCl
Cl∙ + H2 → HCl + H∙
D) Cl2 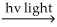 Cl∙ + Cl∙
Cl∙ + Cl∙
Cl∙ + H2 → H∙ + HCl
H∙ + Cl∙ → HCl
Correct Answer:
Verified
Q37: Consider the reaction of A being converted
Q38: In an exothermic reaction, are stronger bonds
Q39: Which of the following correctly expresses the
Q40: Consider the reaction of A being converted
Q41: Predict the enthalpy (ΔH) value for the
Q43: Does one expect ΔS° in a propagation
Q44: If stronger bonds are formed and weaker
Q45: Consider the elementary step in the solvolysis
Q46: The bond dissociation energy is the amount
Q47: Will the sign of ΔS° in the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents