Given the bond dissociation energies below (in kcal/mol) , estimate the ΔH° for the propagation step 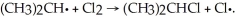 CH3CH2CH2-H 98
CH3CH2CH2-H 98
(CH3) 2CH-H 95
Cl-Cl 58
H-Cl 103
CH3CH2CH2-Cl 81
(CH3) 2CH-Cl 80
A) -22 kcal/mol
B) +22 kcal/mol
C) -40 kcal/mol
D) +45 kcal/mol
Correct Answer:
Verified
Q49: Which compound has the smaller bond dissociation
Q50: Draw the transition state for the hydrogen
Q51: Which of the following is true for
Q52: Predict the signs of ΔH° and ΔS°
Q53: Given the chlorination of acetone shown below,
Q55: What reactive species is produced in the
Q56: Energy is _ when bonds are formed
Q57: Consider the bond dissociation energies listed below
Q58: Of the two C-H bonds shown, which
Q59: The hydrogenation of acetylene to produce ethane
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents