The following reaction occurs readily: 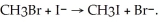
Experimentally one finds that if the concentration of I- is doubled, the rate doubles. Also if the concentration of CH3Br is halved, the rate is halved. What is the rate equation for this reaction?
Correct Answer:
Verified
Q74: Consider the conversion of X to Z
Q75: Given an activation energy of 15 kcal/mol,
Q76: What term describes the highest-energy structure in
Q77: Consider the reaction: CH3CH2∙ + Br2 →
Q78: Consider the three-step mechanism for the reaction
Q80: Provide the structure of the transition state
Q81: Rank the free radicals (I-III) shown below
Q82: When compound I, C7H16, was treated with
Q83: How many distinct dichlorination products can result
Q84: Write the structures of all of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents