Consider the conversion of X to Z through the sole intermediate Y. Given the reaction-energy diagram shown below, which step is the rate-limiting step? Explain your reasoning. 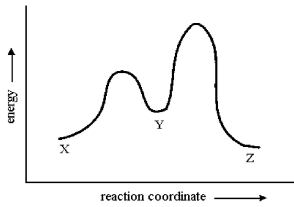
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q69: The Arrhenius equation mathematically models which of
Q70: Consider the one-step conversion of F to
Q71: Rank the following carbocations in order of
Q72: _ is the minimum kinetic energy reacting
Q73: In the hydrocarbon shown below, how many
Q75: Given an activation energy of 15 kcal/mol,
Q76: What term describes the highest-energy structure in
Q77: Consider the reaction: CH3CH2∙ + Br2 →
Q78: Consider the three-step mechanism for the reaction
Q79: The following reaction occurs readily: 
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents