Given an activation energy of 15 kcal/mol, use the Arrhenius equation to estimate how much faster the reaction will occur if the temperature is increased from 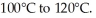
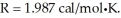
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q70: Consider the one-step conversion of F to
Q71: Rank the following carbocations in order of
Q72: _ is the minimum kinetic energy reacting
Q73: In the hydrocarbon shown below, how many
Q74: Consider the conversion of X to Z
Q76: What term describes the highest-energy structure in
Q77: Consider the reaction: CH3CH2∙ + Br2 →
Q78: Consider the three-step mechanism for the reaction
Q79: The following reaction occurs readily: 
Q80: Provide the structure of the transition state
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents