Remove an H+ from the following structure to create the most reactive (least stable) carbanion. 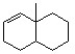
Correct Answer:
Verified
Q116: When the free radical halogenation of methylpropane
Q117: Which of the following reactive intermediate species
Q118: Given that tertiary H atoms react with
Q119: When 1,1,3,3-tetramethylcyclobutane is brominated at 125°C, the
Q120: Draw all monochlorination products expected from reaction
Q122: What reactive intermediate is formed when CHBr3
Q123: Describe the hybridization of the cationic center
Q124: What is the hybridization of the positively
Q125: When acetaldehyde (CH3CHO) is deprotonated, the resulting
Q126: What is the CCC bond angle in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents