Given the following pKa values, what is the major ionization state of histidine at pH 11?
(α-COOH = 2.0, α-NH3+ = 9.0 and R-group imine = 6.5)
A) 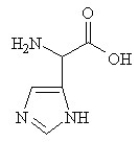
B) 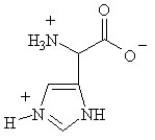
C) 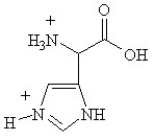
D) 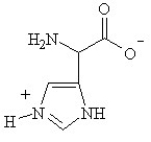
E) 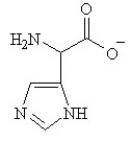
Correct Answer:
Verified
Q43: Which of the following structures is the
Q44: Provide the structure of the predominant form
Q45: What is the isoelectric point of lysine
Q46: Describe how electrophoresis works.
Q47: The isoelectric point is important in _.
A)
Q49: Draw the neutral form of L-phenylalanine in
Q50: Provide a reasonable synthesis of racemic alanine
Q51: What would you start with when making
Q52: Provide a synthesis of racemic valine from
Q53: Provide the structure of the predominant form
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents