The molecule shown below contains ________ sigma bonds and ________ pi bonds. 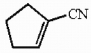
Correct Answer:
Verified
Q67: Explain why the free rotation about the
Q68: From a molecular orbital perspective, why is
Q69: What hybrid atomic orbitals are overlapping to
Q70: Provide the skeletal structures of the five
Q71: Are the two compounds shown below best
Q73: Choose the correct hybridization for the atom
Q74: Are the two compounds shown below best
Q75: From a molecular orbital perspective why isn't
Q76: Which of the following best describes the
Q77: Are the two compounds shown below best
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents