Sodium hydride (NaH) is a base that is commonly used in organic reactions. The pKa of H2 is 36. Which of the following compounds could not be used as a solvent if you were using NaH?
A) 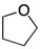
B) 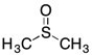
C) CH3CH2OH
D) CH3CH2CH2CH2CH3CH3
Correct Answer:
Verified
Q126: The structure of Tamiflu, an antiinfluenza drug,
Q127: Dopamine is shown below. What functional group,
Q128: Calculate the charge separation for a C-O
Q129: Rank the following compounds in order of
Q130: Use the following structure for the two
Q131: Which molecule below is an alkene?
A) CH3CH2OCH2CH3
B)
Q132: Why doesn't a salt like KCl dissolve
Q133: Use the following structure for the two
Q134: Use the following structure for the two
Q136: Explain why the pKa of acetylene is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents