Classify the compound below as aromatic antiaromatic, or nonaromatic. Assume planarity of the π network. 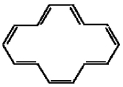
Correct Answer:
Verified
Q29: Classify cycloheptatriene as aromatic, antiaromatic, or nonaromatic.
Q30: Is the [10]annulene shown below aromatic? Explain.
Q31: Aromatic molecules contain _ π electrons.
A) no
B)
Q32: Classify the compound below as aromatic, antiaromatic,
Q33: Which of the following structures is aromatic?
A)
Q35: Which is more stable, cyclobutadiene or 1,3-butadiene?
Q36: Classify the following compound as being aromatic,
Q37: Is the all-cis form of [10]annulene aromatic?
Q38: Provide the structure of tropylium bromide.
Q39: Which of the following structures, if flat,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents